The first section of the AP® Chemistry exam1 consists of 60 multiple-choice questions (MCQs) that account for 50% of your overall score. There are 4 types of MCQs1, each requiring a different approach to answer them correctly. You can benefit from practicing with sample AP Chemistry multiple choice questions to familiarize yourself with the question formats. Our AP Chemistry study guide will also help improve your problem-solving skills.
The question types that you will see on the AP Chemistry exam are:
- Text-based questions
- Diagram-based questions
- Chemical reaction equation questions
- Numerical/mathematical expressions questions
Our AP Chemistry course can help you achieve a high score on the exam. Our realistic practice problems and illustrated explanations are similar to each of the exam's 4 question types.
Text-Based Questions
This category includes both text-based and passage-based questions that require analysis of information presented in written form.
Tips for Answering Text-Based Questions:
- Read questions thoroughly: Look for key terms like “except” or “not” to understand the question's intent.
- Identify relevant concepts: To focus your thinking, recognize the main topic (e.g., equilibrium, or thermodynamics). Apply fundamental rules like periodic trends or Le Chatelier's Principle to guide your answers.
- Eliminate answer choices: Cross out obvious errors to improve your odds with the remaining choices. Choices with “always” or “never” are usually incorrect.
- Trust simple answers: Some questions just test recall; go with your first instinct if confident.
Here is an example of a text-based question:
MCQ 1 Example
A beaker contains 230 g of sucrose in 100 mL of water at 25°C. After stirring the solution, some sucrose dissolves, but some solid sucrose remains at the bottom of the beaker. The beaker is then heated to 90°C and stirred again, and the solid at the bottom of the beaker completely dissolves. Which of the following is true?
- The dissolution of sucrose is endothermic.
- The dissolution of sucrose is exothermic.
- The dissolution of sucrose is endothermic at 25°C and exothermic at 90°C.
- The dissolution of sucrose is exothermic at 25°C and endothermic at 90°C.
What You Must Know:
To correctly answer this question, you need to understand what the terms “endothermic” and “exothermic” mean and be able to decide whether one or both apply to the two scenarios described in the question. To see an example of how UWorld helps you answer this type of question, click here for an illustrated explanation of correct and incorrect answers and the pertinent background information.
Additionally, practicing with our AP Chemistry question bank can help reinforce these concepts through exam-style questions and detailed explanations.
Diagram-Based Questions
Diagram-based MCQs involve visual elements such as diagrams, data tables, graphs, and ranking scenarios. The visuals include all the necessary information to respond to the question.
Tips for Answering Diagram-Based Questions
- Read questions carefully: Mentally note or jot down key terms separately to stay focused on what's asked.
- Examine the diagram closely: Consider critical details, especially any numerical values or labels that might affect your answer.
- Analyze experimental setups: Familiarize yourself with setups like chromatography, electrochemical cells, or titrations, performing calculations as needed.
- Focus on Particle Diagrams: Look at molecule quantities and changes to understand reaction dynamics or states.
- Eliminate Incorrect Choices: Mentally rule out clearly wrong options to narrow down choices.
- Select the Best Answer: Remember that 2 answer options may often seem correct. Use critical thinking to select the option that best aligns with the information in the visual.
Here is an example of a diagram-based question:
MCQ 2 Example
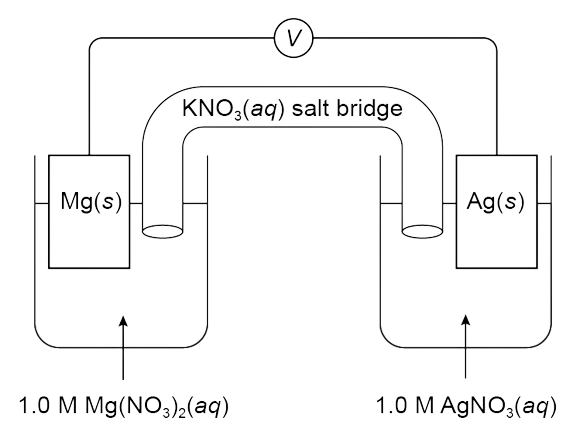
A galvanic cell is constructed with a Mg(s) anode and an Ag(s) cathode and produces an electric current, as represented in the diagram above. Which of the following statements correctly predicts the result of replacing the AgNO3(aq) solution with distilled water and provides the correct explanation?
- The cell will still produce an electric current because the Ag(s) can still be oxidized as the cell operates.
- The cell will no longer produce an electric current because there are no NO3-(aq) ions available to be reduced.
- The cell will no longer produce an electric current because there are no Ag+(aq) ions available to be oxidized.
- The cell will no longer produce an electric current because there are no Ag+(aq) ions available to be reduced.
What You Must Know:
To correctly answer this question, you need to know the purpose of the ionic solution in a galvanic cell and apply this concept to the galvanic cell modification described in the question.
Chemical Reaction Equation Questions
These questions involve reaction equations or structures, requiring you to apply chemistry knowledge accurately.
Tips for Answering Data Table-Based Questions
- Read questions closely: Identify key details and terms to focus on essential information.
- Examine the reaction or structure: Note the types of reactions and any critical details that could influence your answer.
- Perform calculations or manipulations: Complete any necessary calculations, such as balancing equations or mole ratios.
- Eliminate Incorrect Choices: Rule out answers that don't fit based on the reaction or structure provided.
- Choose the best answer: Select the answer that most accurately matches the given information.
Here is an example of a data table-based question:
MCQ 3 Example
6 CO2(g) + 6 H2O(l) → C6H12O6(s) + 3 O2(g)
The formation of C6H12O6(s) and O2(g) during photosynthesis is represented by the equation above. Based on the information in the table below, which of the following is the overall enthalpy of the reaction?
| Substance | ΔH°f (kJ/mol) |
|---|---|
| O2(g) | 0 |
| H2O(l) | −285.8 |
| CO2(g) | −393.5 |
| C6H12O6(s) | −1273 |
- −5349 kJ/molrxn
- −2803 kJ/molrxn
- −593.7 kJ/molrxn
- +2803 kJ/molrxn
What You Must Know:
To correctly answer this question, you need to have a basic understanding of the heat of formation of a compound and how it relates to the enthalpy of a reaction. Use your knowledge and calculation skills to answer this question.
Numerical/Mathematical Expressions Questions
These AP Chem practice MCQs require applying mathematical concepts to find the best answer, including performing calculations or selecting appropriate equations.
Tips for Answering Numerical/Mathematical Expressions Questions
- Read the prompt carefully: Identify and mentally note key details and terms to stay focused.
- Use provided scratch space: For calculations, use available digital tools or scratch paper to stay organized.
- Reference provided resources: Use any periodic tables, contants sheets, and equation sheets offered within the exam.
- Eliminate incorrect choices: Rule out answers that don't align with your calculations.
- Select the best answer: Choose the answer that most closely matches your results.
Here is an example of a numerical/mathematical expressions question:
MCQ 4 Example
P4(s) + 6 Cl2(g) → 4 PCl3(g) ΔH = −2440 kJ/molrxn
P4(s) + 10 Cl2(g) → 4 PCl5(g) ΔH = −3438 kJ/molrxn
Based on the enthalpies for the formation of PCl3(g) and PCl5(g) represented by the reactions above, how much heat is absorbed or released by the decomposition of PCl5(g) represented below?
PCl5(g) → PCl3(g) + Cl2(g) ΔH = ?
- 250 kJ is absorbed
- 998 kJ is absorbed
- 250 kJ is released
- 998 kJ is released
What You Must Know:
To correctly answer this question, you must use basic math skills and understand Hess's law and the enthalpy change in a system.

Frequently Asked Questions (FAQs)
How many multiple-choice questions are on the AP Chemistry exam?
Section 1 of the AP Chemistry exam contains 60 multiple-choice questions that must be answered within 1 hour and 30 minutes.
Can you use a calculator on the MCQ section of the AP Chemistry exam?
Yes. Calculators are allowed on the MCQ section of the exam.
How are AP Chemistry multiple-choice questions graded?
A computer scores the AP Chemistry multiple choice questions section. Learn more about how MCQs are graded, the scoring guidelines, and score distribution by reading our AP Chemistry Score Guide.
How long is the multiple-choice section of the AP Chemistry exam?
You get 1 hour and 30 minutes to answer the multiple-choice questions (MCQ) section, which gives you 1 minute and 30 seconds per question.
When can I get MCQs from previous AP Chemistry exams?
The College Board offers MCQs from previous AP chemistry exams through AP Classrooms. This free medium offers online instructional resources and tools to AP teachers and students regardless of their learning environment.
References
1(2024). 2024-25 AP Chemistry Updates: Overview. AP Educators. College Board. Retrieved on November 01, 2024 from https://www.youtube.com/watch?v=4cwb8WmAZzw
Related Topics
Are you gearing up for the AP Chemistry exam? Check out our guide on exam prerequisites, exam difficulty, topics, and why you should take it.
AP Chemistry Study Guide & MaterialsDo you want to know how to study effectively for the AP Chemistry Exam? Check out this comprehensive study guide to the AP Chem exam designed by our experts.
AP Chemistry Scoring GuideWant to know everything about AP Chemistry Scoring? Here's our easy-to-follow article on scoring and score distribution—including the list of top colleges and their minimum AP Chem score requirements.
How to Self-Study for AP ChemistrySelf-studying for AP Chemistry? Learn how to grasp key concepts, tackle practice problems, and stay on track to ace the AP Chemistry exam!
Best AP Chemistry Study Guide ComparisonNot sure which guide to pick? Read our detailed guide on how Kaplan, Barron's, Princeton Review, and UWorld to find your ultimate prep companion!
Best AP Chemistry Prep Course ReviewWondering which AP Chemistry online course is the best? Check out this detailed review to find the right course for your exam prep.