The AP® Chemistry formula sheet is a 2-page reference guide provided during the exam's multiple-choice (MCQ) and free-response (FRQ) question sections. It includes unit abbreviations, equations, constants, metric prefixes, and definitions of variables organized by topic. You won't have to memorize any of these details, but you should practice with a printed copy of the equation sheet and periodic table of elements to help you apply them efficiently on the exam.
Maximizing the AP Chemistry Formula Sheet
To make your exam prep easier and faster, we've put together an AP Chemistry formula sheet for you to download. Familiarize yourself with the layout by using it during your practice tests, and you'll improve your problem-solving speed and efficiency. For an even more structured review, utilize our AP Chemistry study guide in print and digital formats and try our interactive online course that breaks down complex AP Chem concepts.
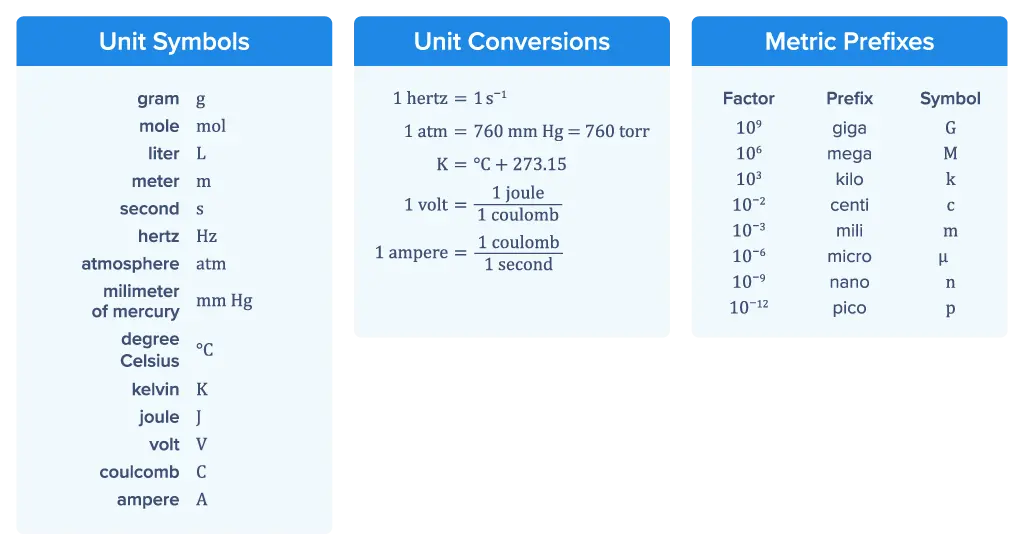
Units at a Glance
The formula sheet includes a list of common AP Chemistry formulas and their units. Understanding units such as meters for length and grams for mass will help you interpret data and solve problems. The sheet also provides unit conversions, along with metric prefixes.
Atomic Structure
Atomic structure explores the makeup of an atom, its electronic structure, and what occurs during electronic transitions when a photon is absorbed or emitted. Equations and constants related to these ideas are provided in the atomic structure section of the AP Chemistry equation sheet. Once you've reviewed the formulas, reinforce your understanding with our AP Chemistry QBank of realistic practice questions and answer explanations.

Equation (1) shows how the energy E of a photon relates to Planck’s constant h, and frequency ν.
Equation (2) relates the speed of light c to wavelength λ and frequency ν.
Equation (3) represents Coulomb’s law, which shows how the electrostatic force Fcoulombic between two charged particles is related to their charges and the distance between them.
Gases, Liquids, and Solutions
Matter exists in solid, liquid, and gas states. Solids can be dissolved in a liquid to make a solution. The different states of matter and solutions exhibit different macroscopic properties such as pressure, density, temperature, and concentration.
The gases, liquids, and solutions section of the formula sheet contains several equations used to calculate different macroscopic properties for gases, liquids, and solutions, as well as the different constants used in these equations.
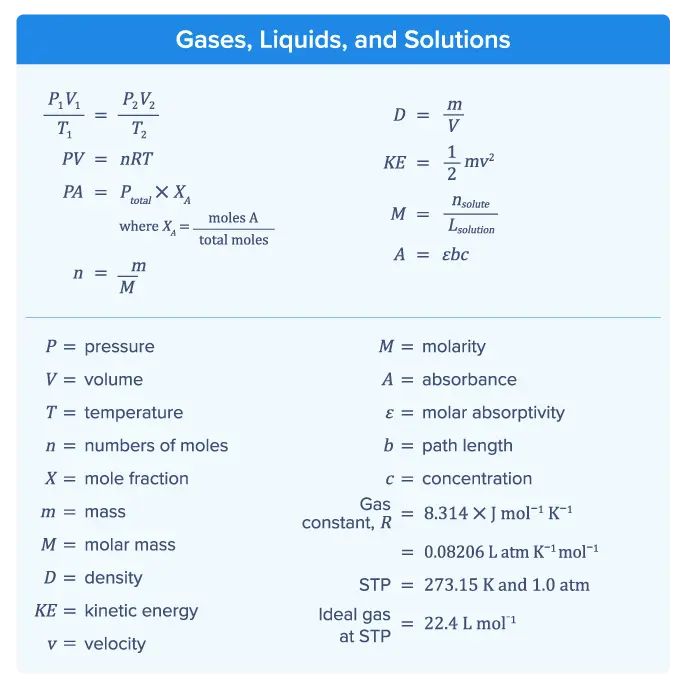
Equation (1) is a form of the Combined Gas Law, which combines Boyle’s Law, Charles’s Law, and Gay-Lussac’s Law. It shows the relationship between the pressure, volume, and temperature of a fixed amount of gas, assuming no gas is added or removed.
Equation (2) is the ideal gas law, which illustrates how an ideal gas behaves and is used to predict how a real gas behaves. The ideal gas law shows that pressure P and volume V are directly proportional to the number of moles of gas n and temperature T.
Equation (3) uses the mole fraction XA and the total pressure Ptotal in a container to calculate the partial pressure of one component PA in the sample.
Equation (4) shows that the total pressure Ptotal in a container is the sum of the partial pressure of each component (PA, PB, etc) in the sample.
Equation (5) shows how to calculate the number of moles n of a substance using the mass m and the molar mass M of that substance. This equation is not limited to being used in gas equations. Several different types of calculations require n, including the concentration of a solution.
Equation (6) indicates how mass m and velocity v are used to calculate the kinetic energy KE of a molecule.
Equation (7) shows that the density D of a substance is a ratio of mass m to volume V.
Equation (8) shows that kinetic energy (KE) is equal to half of the object’s mass (m) multiplied by the square of its velocity (v).
Equation (9) in this section is Beer’s Law, which is used in UV-vis spectroscopy (measuring the absorbance of UV-vis light by a solution at different wavelengths). Beer’s Law describes the relationship between the absorbance A, molar absorptivity ε, path length b of the container through which the light passes, and the concentration of the solution c.
Three values of the gas constant R (used in the ideal gas law) are given. The only difference between each value is the units of pressure. You can use any of these values in the ideal gas law equation as long as the units of pressure for the value of R used match the units of pressure for P.
The conditions of STP (standard temperature and pressure) and the molar volume of an ideal gas at STP are also given.
Kinetics
Kinetics focuses on reaction rate, or how fast a chemical reaction occurs. The rate law describes the reaction rate. The rate constant k used in rate law expressions is calculated by measuring the change in reactant concentration over time. The data obtained from measuring how reactant concentration changes over time are plotted in 3 ways. One of the 3 graphs will be linear, indicating the reaction order.
The kinetics section of the AP Chemistry equation sheet provides different equations you can use to calculate the rate constant k, depending on the order of the reaction.
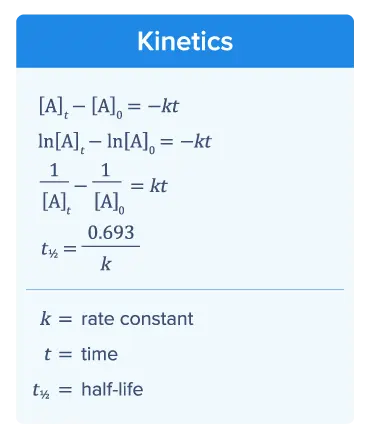
Equation (1) allows for the calculation of 𝑘 in zero-order reactions.
Equation (2) enables the calculation of 𝑘 in first-order reactions.
Equation (3) facilitates the calculation of 𝑘 in second-order reactions.
The half-life t1/2 of a reaction is the amount of time it takes for the concentration of the reactants to be reduced by one-half.
Equation (4) allows you to calculate 𝑘 for first-order reactions if you know the t1/2 of the reaction.
Equilibrium
Equilibrium focuses on reversible reactions, including some acid-base reactions, and how changing reaction conditions affects the direction of the reaction. Performing calculations involving the equilibrium constant K and understanding what the magnitude of K implies about equilibrium concentrations are essential for success on the AP Chemistry exam.
The equilibrium section of the formula sheet provides several equations necessary for problems involving equilibrium or acid-base chemistry.
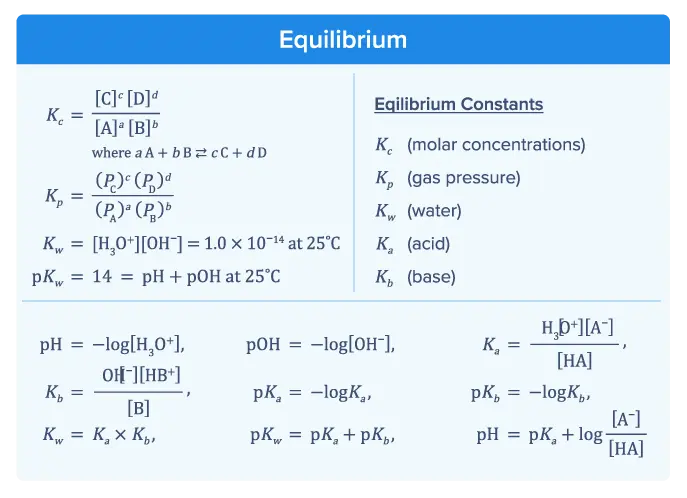
- Kc represents the equilibrium constant in terms of molar concentrations of reactants and products. For a general reaction where reactants A and B form products C and D.
- [A], [B], [C], and [D] are the molar concentrations of the reactants and products, and a, b, c, and d are their respective stoichiometric coefficients.
- The equation shows that the equilibrium constant is the ratio of the product concentrations raised to their respective powers divided by the reactant concentrations raised to their respective powers.
- Kp is the equilibrium constant in terms of partial pressures for gases.
- PA, PB, PC, and PD represent the partial pressures of the reactants and products, and a, b, c, and d are their respective stoichiometric coefficients.
- Kw is the ion product of water, representing the equilibrium constant for the dissociation of water into hydronium ions H3O+ and hydroxide ions OH–.
- The value 1.0×10-14 is specific at 25°C and shows the product of these ion concentrations at equilibrium.
- This equation shows that at 25°C, the sum of pH (which measures the acidity of a solution) and pOH (which measures the basicity of a solution) always equals 14.
- This equation shows that pH is the negative logarithm of the hydronium ion concentration [H3O+].
- This equation shows that pOH is the negative logarithm of the hydroxide ion concentration [OH–].
- Ka is the equilibrium constant for the dissociation of an acid, where [HA] is the concentration of the acid, and [H3O+] and [A-] are the concentrations of the hydronium ions and conjugate base, respectively.
- Kb is the equilibrium constant for the dissociation of a base, where [B] is the concentration of the base, and [OH-] and [HB+] are the concentrations of hydroxide ions and conjugate acid, respectively.
- pKa is the negative logarithm of the acid dissociation constant, which helps determine the strength of an acid.
- pKb is the negative logarithm of the base dissociation constant, which helps determine the strength of a base.
- This equation shows the relationship between Kw (the dissociation constant of water), Ka (the dissociation constant of an acid), and Kb (the dissociation constant of a base). At equilibrium, the product of Ka and Kb equals Kw.
- This is the Henderson-Hasselbalch equation, which is used to calculate the pH of a buffer solution.
- pKa is the negative logarithm of the acid dissociation constant, and the ratio [A–] / [HA] represents the concentrations of the conjugate base and the weak acid, respectively.
Thermodynamics/Electrochemistry
Thermodynamics focuses on the relationship between Gibbs free energy G, enthalpy H, and entropy S, and how the change in standard Gibbs free energy ΔG° relates to the favorability of a chemical reaction.
Electrochemistry covers galvanic and electrolytic cells, how to determine the standard cell potential E° and free energy of the cell, and how to apply the Nernst equation and Faraday's law.
The final section of the AP Chemistry formula sheet contains several equations and constants used in problems related to thermodynamics and electrochemistry.
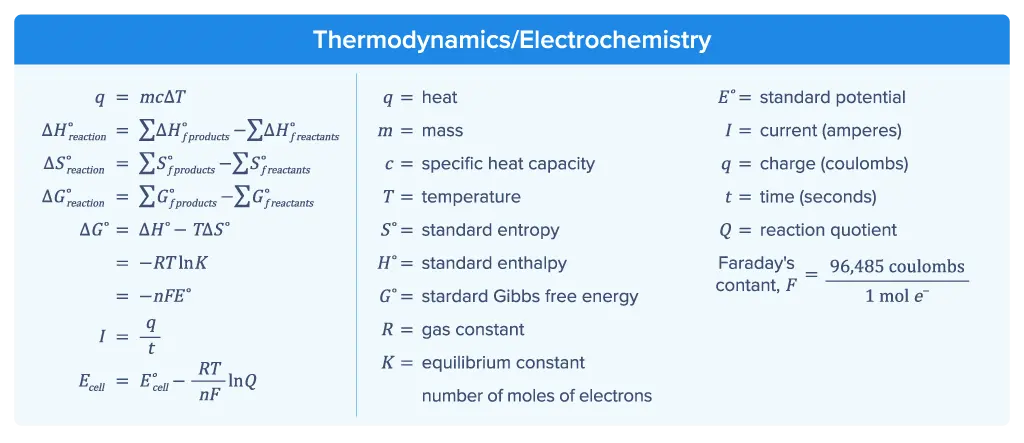
Equation (1) q = mcΔT is used to determine the amount of heat q absorbed or released by a substance. It requires knowing the mass of the substance m, specific heat capacity of that substance c, and the change in temperature ΔT (i.e., Tfinal − Tinitial) in ℃. This equation is often used in calorimetry problems.
Equation (2) is used to calculate the standard enthalpy change ΔH° for a reaction. ΔH° is determined by subtracting the sum of the standard enthalpies of formation of the products ΔHf°products from the sum of the standard enthalpies of formation of the reactants ΔHf°reactants.
Equation (3) shows that the standard entropy change ΔS° for a reaction is calculated by subtracting the sum of the standard entropies of the products S°products from the sum of the standard entropies of the reactants S°reactants.
Equation (4) provides the calculation for the standard Gibbs free energy change ΔG°reaction. This is found by subtracting the sum of the standard free energy of formation of the reactants Σ ΔG°f reactants from the sum of the standard free energies of formation of the products Σ ΔG°f products.
Equation (5) relates the standard Gibbs free energy change ΔG° to the changes in enthalpy ΔH°, entropy ΔS°, and temperature ΔT, the equilibrium constant K, the number of moles of the electrons n, Faraday’s constant F, and the standard cell potential E°
Equation (6) gives the relationship between current I, charge q, and time t. This equation is useful in electrolysis calculations and in applying Faraday’s law.
Equation (7) is the Nernst equation, which determines Ecell under non-standard conditions (standard conditions are 1 M solution concentration, system pressure of 1 atm, and temperature of 25 ℃).
Faraday’s constant F, which is used in some calculations of ΔG° and the Nernst equation, is given.
The relationship between volts, joules, and coulombs is given.
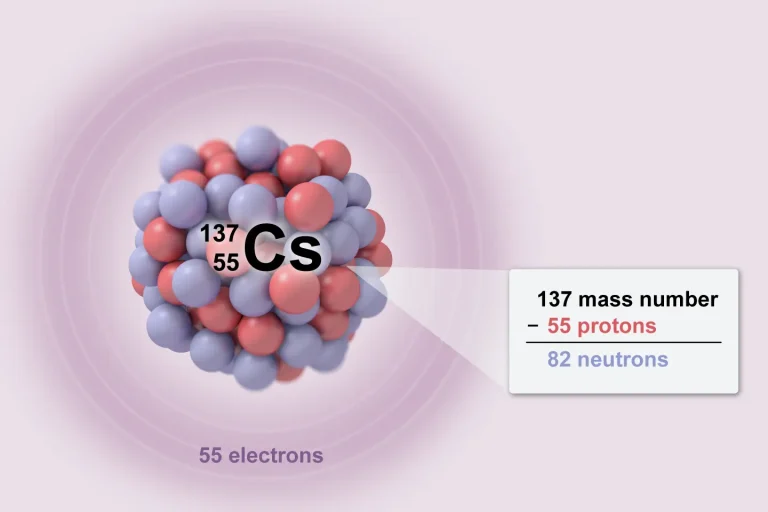
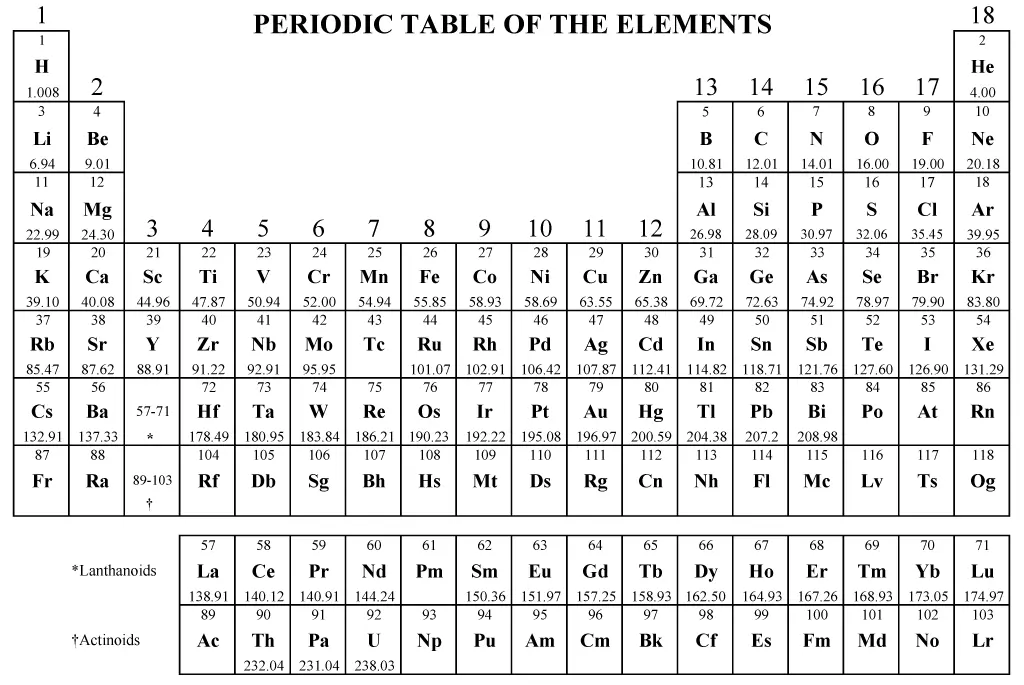
Periodic Table of Elements
You will be provided a periodic table for your AP Chemistry exam. This reference table can be used to solve various chemistry problems. Each element on the periodic table has information including atomic number, symbol, atomic mass, and sometimes other properties such as electronegativity. Knowing how to use the periodic table effectively will be a big advantage on the exam.
Key Features to Know:
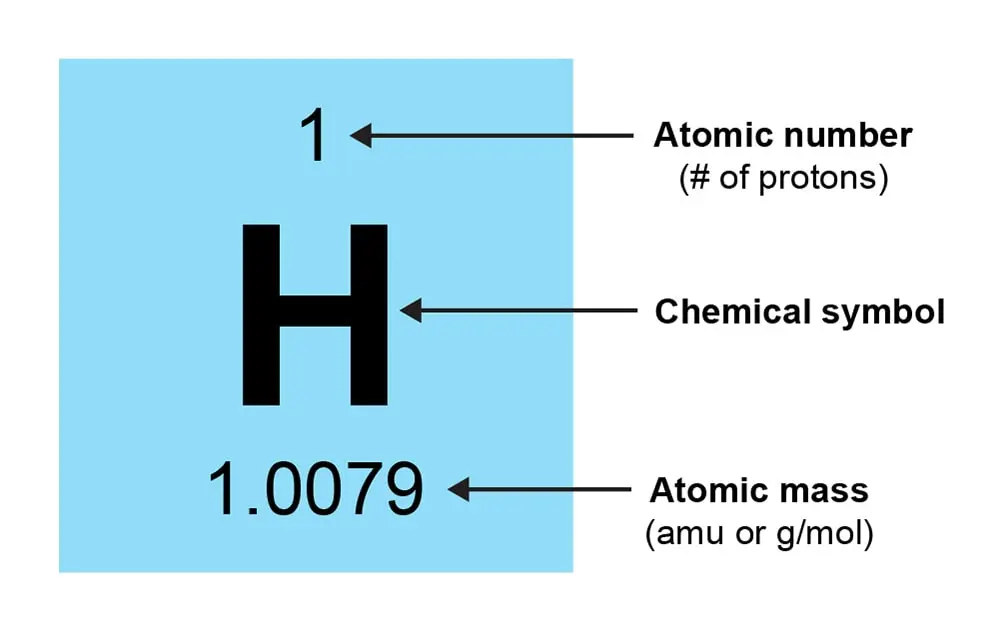
Located at the top of the square, indicating the number of protons in an atom of the element.
The abbreviation of the element’s name in the center.
Found below the element symbol, representing the average mass of an atom.
These properties may be indicated and are vital for predicting chemical behavior.
Located at the top of each column on the periodic table.

References
- (n.d.). AP Chemistry Equations & Constants.Oklahoma State University. Retrieved on March 24,2025 from https://intro.chem.okstate.edu/Arkansas/Chem%20CB%20Formula%20Sheet.pdf
- (n.d.). Periodic Table Of the Elements. AP Chemistry Course and Exam Description. College Board. Retrieved on March 24, 2025 from https://apcentral.collegeboard.org/media/pdf/ap-chemistry-course-and-exam-description.pdf
Read More About AP Chemistry
Want to get a better sense of the AP Chem course and exam? We've compiled a list of frequently asked questions and valuable information about AP Chemistry.
AP Chemistry Exam FormatThe format of the AP exam can be confusing to understand. Check out our article on the AP Chemistry Exam Format, which explains the exam format, question types, and more!
AP Chem Course and Exam DescriptionWant to know what each unit, topic, and concept looks like in AP Chemistry? Learn about each of the 9 units, 4 big ideas, and 6 labs clearly in our AP Chemistry CED.
How to Self-Study for AP ChemistrySelf-studying for AP Chemistry? Discover tips on mastering concepts, tackling practice problems, and staying organized to excel on the AP Chemistry exam!
Best AP Chemistry Study Guide ComparisonStruggling to choose the best study guide? Compare Kaplan, Barron's, Princeton Review, and UWorld to find the perfect AP Chemistry study guide for your exam success!
Best AP Chemistry Prep Course ReviewSearching for top AP Chemistry online courses? Read this review to compare the best courses and choose the perfect one for your success.