AP® Chemistry Unit 9 Review and Practice Test
Get ready to ace AP® Chemistry Unit 9: Thermodynamics and Electrochemistry with UWorld’s expertly crafted review. We make challenging topics clear and engaging through visuals and detailed walkthroughs. Then, apply your knowledge with AP Chemistry Unit 9 progress check MCQs and FRQs that simulate real exam conditions. Each explanation will strengthen your grasp of energy and redox principles so you can approach every AP Chem Unit 9 question with confidence and accuracy.
Boost Your Confidence and Score High with Our AP Chemistry Unit 9 Review
Turn AP Chem Unit 9 stress into success! UWorld’s smart, structured review combines a comprehensive study guide, an extensive question bank, and engaging video lessons to simplify thermodynamics and electrochemistry. Strengthen your understanding, sharpen your problem-solving, and set yourself up to score a solid 5 on exam day.
Engaging Video Lessons
Learning doesn’t have to feel like a chore. UWorld’s interactive video lessons keep you engaged and motivated while simplifying even the hardest chemistry concepts. Watch, learn, and get one step closer to acing your Unit 9 AP Chemistry test.
Interactive Study Guides
Study smarter with UWorld’s study guide, created to mirror the College Board® CED. It combines concise notes, labeled visuals, and practice checkpoints that turn review time into real progress toward a 5 on the AP Chem exam.
Try These AP Chemistry Unit 9 Practice Test Questions
Question
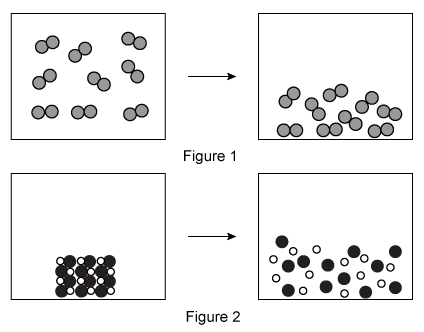
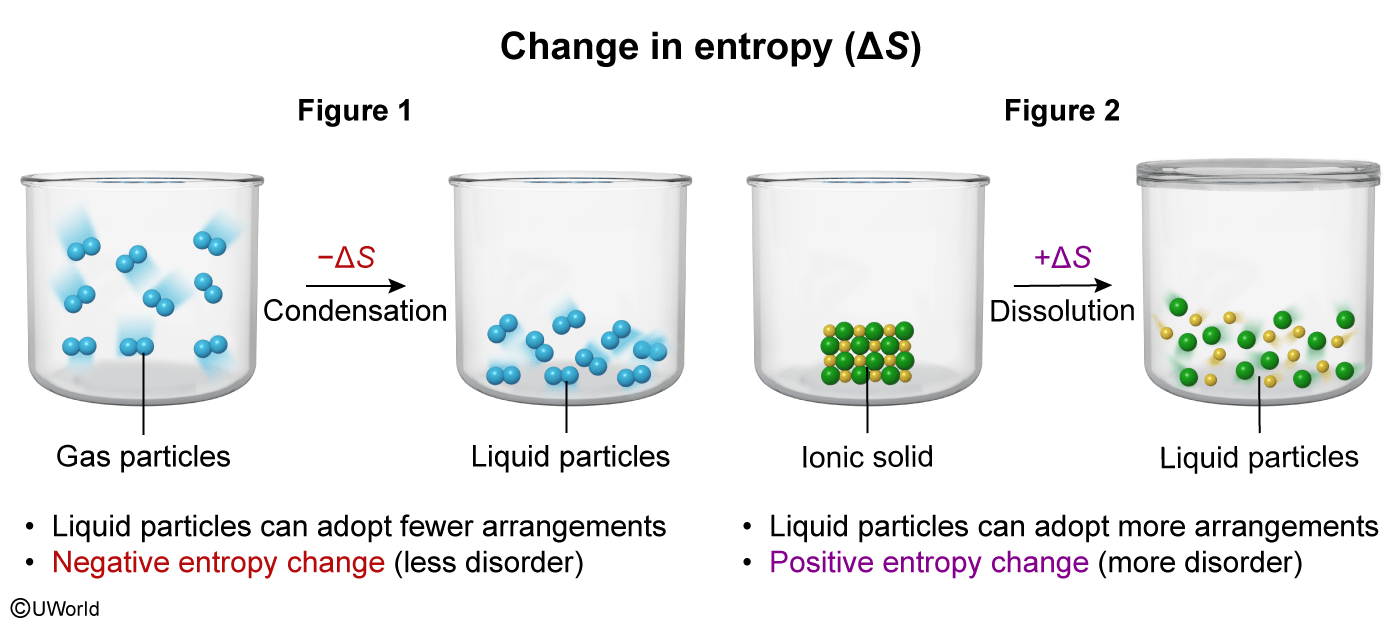
The particulate diagram in Figure 1 above shows a gas condensing into a liquid, and the particulate diagram in Figure 2 shows an ionic solid being dissolved in aqueous solution. Which figure depicts a negative entropy change, and why?
| A. Figure 1, because the liquid particles can adopt more arrangements than the gas particles. | |
| B. Figure 1, because the liquid particles can adopt fewer arrangements than the gas particles. | |
| C. Figure 2, because the ions in solution can adopt more arrangements than the particles in the solid. | |
| D. Figure 2, because the ions in solution can adopt fewer arrangements than the particles in the solid. |
Explanation
Entropy indicates the molecular disorder of a system. A process resulting in a positive entropy change (ΔS > 0) indicates the system became more disordered and can adopt more arrangements whereas a process resulting in a negative entropy change (ΔS < 0) indicates the system became more ordered and can adopt fewer arrangements.
Matter commonly exists in three phases: solid, liquid, or gas. The characteristics of particles in each phase determine its relative entropy:
-
Gas particles have the greatest entropy because they are spaced far apart, can move freely, and can adopt a greater number of arrangements than particles in solids and liquids.
-
Liquid particles are held closely together by intermolecular attractions and can form fewer possible arrangements than gas particles.
-
Solid particles have the lowest entropy because they are closely packed and held together in a fixed arrangement.
The question states that Figure 1 shows a condensation (ie, transitioning from a gas to a liquid). This results in a decrease in entropy because liquid particles can adopt fewer arrangements than gas particles. Therefore, Figure 1 depicts a negative entropy change.
(Choice A) Liquid particles are packed more closely than gas particles and can form fewer arrangements than gas particles.
(Choices C and D) Figure 2 shows dissolution of an ionic solid. Ions in solution can adopt more arrangements than particles in the solid (ie, Figure 2 depicts a positive entropy change).
Things to remember:
Entropy indicates the molecular disorder of a system. Negative entropy changes (a decrease of entropy) result in a system that can adopt fewer arrangements. Positive entropy changes result in more arrangements.
Question
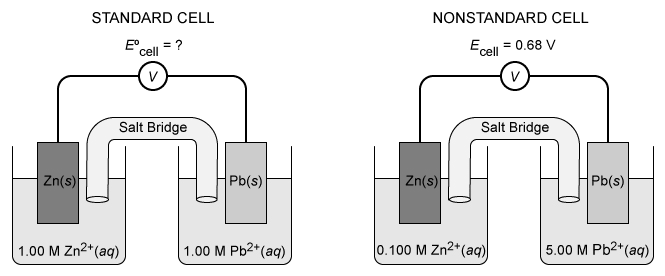
A standard and a nonstandard variation of a galvanic cell are constructed, as shown above. The nonstandard cell produces a potential of 0.68 V at 25 °C. According to the Nernst equation, the potential produced by the standard cell must be:
| A. less than 0.60 V. | |
| B. greater than 0.60 V but less than 0.68 V. | |
| C. 0.68 V. | |
| D. greater than 0.68 V. |
Explanation
In a galvanic cell, a favorable electrochemical reaction that is not at equilibrium drives the production of an electric current. Because the cell reaction is not at equilibrium, Le Châtelier's principle cannot be applied to describe changes in electrochemical cells. These cells are instead described by the Nernst equation:
where Ecell is the observed cell voltage, E°cell is the cell voltage under standard conditions, Q is the reaction quotient, and the other variables are constants and reaction parameters.
Four cases are possible for an electrochemical cell:
| At equilibrium, Q is equal to the equilibrium constant Keq and Ecell = 0 |
| Under standard conditions, where lnQ = 0, Ecell = E°cell. |
| For nonstandard conditions with a small, positive Q, Ecell > E°cell. |
| For nonstandard conditions with a larger, positive Q, Ecell < E°cell. |
For the electrochemical cells in this question, the following redox reactions take place:
Applying the molar concentrations given for the nonstandard cell, its reaction quotient is:
Because Q < 1, Ecell produced by the nonstandard cell must be greater than E°cell produced by the standard cell. The exact value is determined by substituting the values of Ecell and Q into the Nernst equation and solving for E°cell:
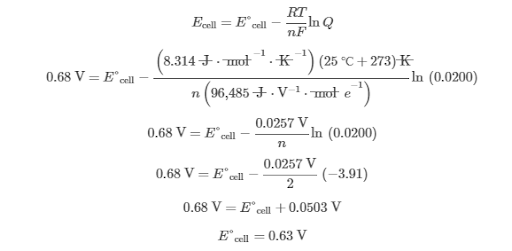
Therefore, the potential produced by the standard cell is greater than 0.60 V but less than0.68 V.
(Choice A) Inverting the ratio as during the calculation yields a result of 0.58 V.
(Choice C) Because Q < 1, E°cell cannot be equal to Ecell.
(Choice D) E°cell cannot be greater than Ecell unless Q > 1.
Things to remember:
The Nernst equation describes the potential Ecell of a galvanic cell relative to the standard cell potential E°cell for a given reaction quotient Q. Four cases are possible:
- Q = Keq with Ecell = 0
- Q = 1 with Ecell = E°cell
- Q < 1 with Ecell > E°cell
- Q > 1 with Ecell < E°cell
Question
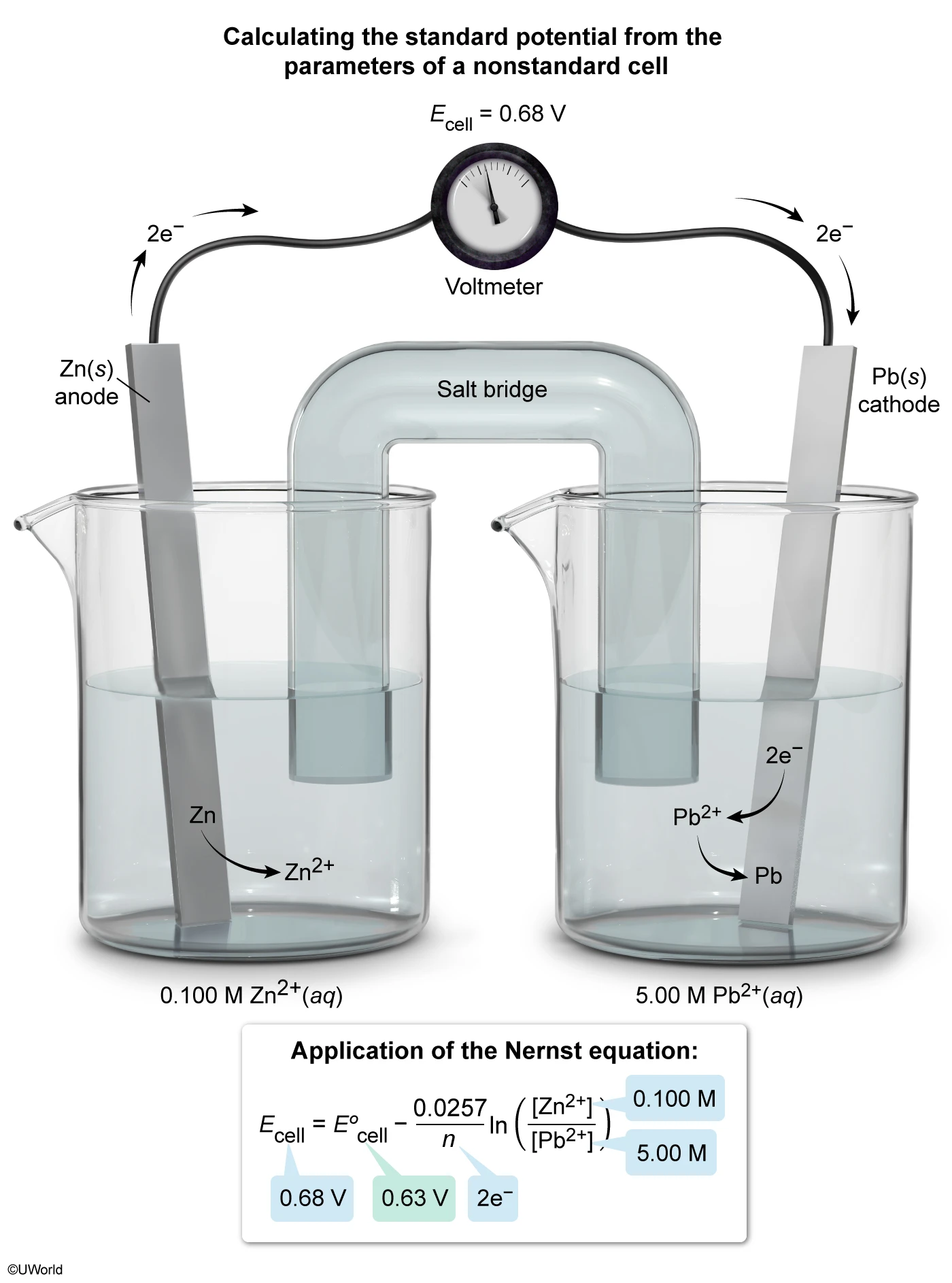
A student dissolves a sample of KBr(s) in a beaker filled with water at 25 °C. During the dissolution process, interactions between the solute ions are broken and interactions between the solute ions and solvent molecules are formed. Which of the following statements correctly describes the contribution to the overall change in entropy caused by breaking and forming these interactions?
| A. Breaking solute-solute interactions increases the entropy whereas forming solute-solvent interactions decreases the entropy. | |
| B. Breaking solute-solute interactions decreases the entropy whereas forming solute-solvent interactions increases the entropy. | |
| C. Breaking solute-solute interactions and forming solute-solvent interactions both increase the entropy. | |
| D. Breaking solute-solute interactions and forming solute-solvent interactions both decrease the entropy. |
Explanation
Entropy is a measure of the molecular disorder, or randomness, of a system. Solids (eg, ionic compounds) contain atoms that are held in a fixed arrangement with little room to move; consequently, solids have relatively low entropy. However, liquids (eg, water) have higher entropy because the atoms in liquids are free to move into many random arrangements.
The process of dissolving an ionic solid (solute) in water (solvent) occurs by simultaneous steps that each involve either an increase or decrease in entropy:
-
Entropy increases when breaking solute-solute interactions (eg, electrostatic attractions between ions), allowing the solute to randomly disperse in the solvent.
-
Entropy increases when breaking solvent-solvent interactions (eg, hydrogen bonding) between the water molecules are broken (ie, allowing greater freedom of movement) so that new solute-solvent interactions can be formed.
-
Entropy decreases when forming new solute-solvent interactions (eg, ion-dipole interactions) between the solute ions and water molecules, causing diminished motion and less disorder.
In this question, dissolving the ionic compound KBr(s) in water involves breaking the solute-solute interactions between the K+ and Br− ions (ie, increases entropy) and forming new solute-solvent interactions between the ions and water molecules (ie, decreases entropy).
(Choices B and C) Forming solute-solvent interactions restricts molecular motion and decreases entropy.
(Choice D) Breaking solute-solute interactions allows molecular motion and increases entropy.
Things to remember:
Entropy describes the disorder (randomness) of a molecular system. Entropy increases when solute-solute interactions and solvent-solvent interactions are broken and decreases when solute-solvent interactions are formed.
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chem Unit 9 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 9: Thermodynamics and Electrochemistry?
Unit 9 of the AP Chemistry curriculum focuses on thermodynamics and electrochemistry, exploring how energy changes drive chemical reactions and how chemical reactions generate or consume electricity. Key topics include:
- Introduction to Thermodynamics: Explores the fundamental concepts of energy, heat, and work in chemical systems.
- Enthalpy: Focuses on heat changes at constant pressure and their implications in chemical reactions.
- Entropy: Discusses the measure of disorder or randomness in a system and its role in spontaneous processes.
- Gibbs Free Energy: Introduces the criterion for spontaneity of processes and how it combines enthalpy and entropy.
- Electrochemical Cells: Covers the conversion of chemical energy into electrical energy and vice versa, including galvanic and electrolytic cells.
- Standard Electrode Potentials: Examines the voltage associated with half-reactions and their use in predicting the direction of redox reactions.
- Nernst Equation: Applies the relationship between concentration and electrode potential to determine cell potential under non-standard conditions.
- Electrolysis: Explores the process of driving a non-spontaneous reaction using electrical energy.
- Applications of Electrochemistry: Investigates real-world applications such as batteries, corrosion, and electroplating.
- Thermodynamic vs. Kinetic Control: Differentiates between reactions controlled by thermodynamic stability and those controlled by the rate of reaction.
These topics form the basis for Unit 9 progress check MCQ AP Chemistry answers and are essential to review using AP Chem Unit 9 practice questions. UWorld’s review tools, including quizzes and interactive study guides, make it easy to master these concepts efficiently and prepare for both MCQs and FRQs.
How should I prepare for an AP Chemistry Unit 9 exam?
Preparing for AP Chem Unit 9 requires a structured approach that balances understanding concepts, practice, and review. Here’s how:
- Build a Strong Conceptual Foundation: Start with UWorld’s interactive study guide and video lessons to understand core ideas in thermodynamics and electrochemistry. Visual explanations and step-by-step lessons help you retain complex concepts.
- Practice with Exam-Style Questions: Work through Unit 9 progress check MCQ AP Chemistry answers and practice FRQs to reinforce problem-solving skills and apply concepts in exam-style scenarios.
- Review Detailed Rationales: For every practice question, study the rationales behind why each answer is correct or incorrect. This helps reinforce understanding and clarify tricky topics.
- Identify and Focus on Weak Areas: Track your performance in quizzes and practice tests. Spend extra time on topics where you struggle, using targeted lessons and review materials.
- Simulate Real Exam Conditions: Take timed Unit 9 practice tests to build test-taking speed and confidence. This also helps manage time and reduce anxiety on exam day.
- Consistent Review and Reinforcement: Revisit challenging concepts regularly. Combining active learning with repeated practice ensures mastery and prepares you to aim for a solid 5.
Are any free resources available for AP Chemistry Unit 9?
Yes! UWorld offers a free trial that gives access to a selection of AP Chemistry Unit 9 MCQ practice questions and interactive tools. You can explore multiple-choice and free-response questions, try the study guides and video lessons, and get a sense of how the platform helps reinforce key thermodynamics and electrochemistry concepts before subscribing.
In addition, other free resources like College Board materials and Khan Academy can complement your prep. The College Board provides the official exam framework and sample questions, while Khan Academy strengthens conceptual understanding. When combined with UWorld’s realistic Unit 9 practice questions and detailed rationales, these resources let you study efficiently, target areas of improvement, and connect foundational knowledge to real exam scenarios, helping you improve comprehension and aim for a solid 5.
What types of questions are on the AP Chemistry Unit 9 test?
The AP Chemistry Unit 9 exam assesses both content knowledge and your ability to apply concepts to new situations. Question types include:
- Multiple-Choice Questions (MCQs): These may be discrete questions or grouped in sets with a stimulus, such as a data table, graph, or experimental scenario, followed by related questions. They assess your ability to analyze information, apply concepts, and solve problems efficiently.
- Free-Response Questions (FRQs): Typically, there are 3 long-answer and 4 short-answer questions. Long-answer questions are worth 10 points each, while short-answer questions are worth 4 points. FRQs test your ability to construct explanations, perform calculations, represent data, and use scientific reasoning.
- Data Analysis & Interpretation: Questions often include graphs, experimental results, or reaction scenarios that require you to interpret, compare, or predict outcomes based on chemical principles.
- Mathematical & Conceptual Reasoning: Expect problems requiring calculations of energy changes, cell potentials, or reaction spontaneity, often combined with conceptual explanations.
These question formats mirror the AP exam’s balance of conceptual understanding and application skills. UWorld’s Unit 9 question bank covers all these formats with realistic MCQs and FRQs, complete with detailed rationales for each answer choice. Practicing with these tools helps you become familiar with the style and difficulty of the real AP Chemistry test and ensures you’re ready to tackle anything that appears on exam day.
What are the common mistakes students make in AP Chem Unit 9?
Many students struggle with thermodynamics and electrochemistry because they focus on memorizing formulas rather than understanding the underlying concepts. Common errors include misinterpreting Gibbs free energy, enthalpy, and entropy relationships, confusing cell notation and electrode potentials, or forgetting to account for standard versus non-standard conditions in calculations.
Another frequent mistake is underestimating the importance of practice and application. Students often skip solving enough AP Chemistry Unit 9 progress check FRQ or Unit 9 AP Chemistry MCQ, which can lead to weak problem-solving skills and lower confidence during the exam. Inconsistent review of concepts and neglecting to connect theory to real experimental scenarios also contributes to avoidable errors. Using structured resources like UWorld’s Unit 9 AP Chem Unit 9 review, including study guides, video lessons, and question bank, helps students identify weak spots, reinforce understanding, and reduce mistakes on test day.
How do I self-study for AP Chemistry Unit 9 effectively?
Effective self-study for AP Chem thermodynamics combines concept mastery with targeted practice. Begin with UWorld’s interactive study guides and video lessons to understand key ideas in AP chemistry electrochemistry, thermodynamics, and energy changes. Take organized notes to summarize formulas and relationships, focusing on comprehension rather than rote memorization.
Reinforce learning by practicing AP Chemistry Unit 9 MCQ and FRQ problems from UWorld’s question bank. Review the rationales for each answer to strengthen your skills and deepen understanding. Simulate timed conditions to improve speed and build confidence. Consistent review and active engagement help retain knowledge and maximize your chances of scoring a solid 5 on the AP exam.
How can I improve my score on the Free-Response Questions (FRQs) for Unit 9?
Improving your AP Chemistry Unit 9 FRQ performance starts with focused, consistent practice. Review past questions to understand how AP Chem Unit 9 topics in thermodynamics and electrochemistry are tested and learn the structure of high-scoring responses. Clearly show your reasoning, include correct units, and explain each calculation step to demonstrate a solid grasp of underlying concepts.
This winter, UWorld is launching AP Chemistry Unit 9 progress check FRQ practice questions with AI-based scoring method to replicate real exam scenarios and include detailed feedback, highlighting what AP graders look for. Pairing consistent practice with guided explanations helps refine your answers, improve accuracy, and build confidence, ensuring you’re fully prepared to tackle any FRQ on the Unit 9 exam.
What is the "Thermodynamics and Electrochemistry" unit's weight on the AP Chemistry exam?
While the exact weighting can vary slightly, Unit 9 AP Chemistry: Thermodynamics and Electrochemistry generally accounts for 7–9% of the total AP Chemistry exam score. Teachers typically spend 10–13 class periods covering this unit, as it includes high-yield topics that frequently appear in both Unit 9 AP Chemistry MCQ and AP Chemistry Unit 9 progress check questions.
A strong understanding of enthalpy, entropy, Gibbs free energy, and electrochemical cells is essential for success. These concepts are often tested in data interpretation and problem-solving scenarios. Using UWorld’s AP Chem Unit 9 review, targeted question bank, and FRQ practice allows you to focus efficiently, reinforce critical areas, and master this unit to maximize your overall exam performance.
Where can I find a good study guide for AP Chemistry Unit 9?
An effective AP Chem Unit 9 study guide should combine conceptual learning with active practice. UWorld’s digital and print Unit 9 AP Chemistry review materials cover every learning objective in thermodynamics and electrochemistry, featuring clear explanations, labeled visuals, and knowledge checks after each section. Instead of just reading, you’ll complete practice questions to confirm understanding before moving on.
For on-the-go study, the UWorld mobile app provides digital notes, flashcards, and a customizable study planner. You can even start with a free trial to explore how UWorld’s integrated approach “Read, Watch, and Practice” compares to other AP Chemistry Unit 9 review resources. By combining interactive content with exam-style questions, UWorld helps you master challenging concepts quickly and prepares you to aim for a solid 5 on the AP exam.
Can I find practice tests specifically for AP Chem Unit 9?
Absolutely! UWorld offers customizable AP Chemistry Unit 9 practice tests that let you focus on thermodynamics and electrochemistry topics with precision. These practice tools are designed to mirror real exam conditions and help you learn actively, not just review passively.
- Topic-wise practice: Target specific areas, such as Gibbs free energy, entropy, or electrochemical cells, to strengthen your areas of improvement.
- Unlimited attempts: Revisit practice questions as often as you need until you master each concept.
- Exam-level difficulty: Questions replicate the tone, structure, and rigor of actual AP Chemistry exam items.
- Instant feedback: Detailed rationales explain why every answer choice is right or wrong, deepening conceptual understanding.
- Progress tracking: Monitor performance trends to fine-tune your prep strategy and focus efficiently.
With UWorld’s full AP Chem Unit 9 course, you’ll gain access to all Unit 9 practice tests, interactive study guides, and expert video lessons. This structured, all-in-one prep system ensures you study smarter, build lasting confidence, and get one step closer to scoring a solid 5 on the exam.
What are the Unit 9 topics most frequently tested on the AP Chemistry exam?
Before taking the AP Chemistry Unit 9 test or your final exam, make sure you’re confident in the key thermodynamics and electrochemistry concepts that frequently appear on the AP exam.
- Thermodynamic principles: Understanding the relationships between enthalpy, entropy, and Gibbs free energy and how they determine spontaneity.
- Enthalpy changes and calorimetry: Calculating energy transfer using heat flow and thermochemical equations.
- Entropy and the second law of thermodynamics: Predicting disorder changes and connecting them to system spontaneity.
- Gibbs free energy calculations: Applying ΔG = ΔH – TΔS to determine reaction favorability under different conditions.
- Electrochemical cells: Analyzing galvanic and electrolytic cells, identifying anodes, cathodes, and direction of electron flow.
- Standard reduction potentials: Using half-cell reactions to calculate cell potentials and assess reaction feasibility.
- Nernst equation and non-standard conditions: Adjusting E°cell values for temperature and concentration changes.
What is the best way to review AP Chemistry Unit 9 before the AP exam?
A strong AP Chemistry Unit 9 review blends conceptual learning, visual understanding, and consistent practice. UWorld combines all these strategies in one platform to help you confidently master thermodynamics and electrochemistry before exam day.
- Read: Begin with UWorld’s AP Chemistry Unit 9 study guide, featuring concise explanations and diagrams that simplify concepts like enthalpy, entropy, Gibbs free energy, and electrochemical cells.
- Watch: Explore high-yield video lessons with visual breakdowns of reaction spontaneity, energy flow, and redox reactions in real-world systems.
- Practice: Reinforce concepts using UWorld’s AP Chem Unit 9 question bank, where each item includes step-by-step rationales and visuals that strengthen problem-solving skills.
- Active Recall & Spaced Repetition: Use customizable quizzes and flashcards to revisit key thermodynamic formulas and oxidation-reduction patterns at spaced intervals for long-term retention.
- Timed Practice: Simulate exam conditions with Unit 9 practice tests in UWorld’s test mode to build speed, confidence, and endurance.
- Targeted Review & Tracking: Review analytics in UWorld’s performance dashboard to identify weak areas, revisit challenging topics, and measure improvement.
With UWorld’s all-in-one system, you can move seamlessly from studying energy transformations to mastering AP Chemistry Unit 9 MCQ and FRQ practice, ensuring complete preparation for both Unit 9 assessments and the full AP Chemistry exam.