AP® Chemistry Unit 7 Review and Practice Test
Master AP Chemistry equilibrium with our Unit 7 review. Learn key concepts, tackle practice questions, and get expert tips to strengthen your understanding before exam day.
AP Chem Unit 7 Review: Conquer Equilibrium Like a Pro
Prepare for your AP Chemistry Unit 7 progress; check MCQ with in-depth explanations, step-by-step examples, and expert study tips. Strengthen your understanding of equilibrium concepts and gain the confidence to answer every question accurately.
Engaging Video Lessons
Watch AP Chemistry Unit 7 MCQ concepts come to life through short, engaging video lessons. Experts simplify tough equilibrium topics, like Le Châtelier’s principle and equilibrium constants, with clear visual explanations. Perfect for quick reviews or in-depth study sessions before your exam.
Interactive Study Guides
Simplify your AP Chem prep with interactive study guides that make learning equilibrium easy. Explore key formulas, graphs, and examples in a clear, step-by-step format. Perfect for reviewing difficult concepts and building confidence before the test.
Try These AP Chemistry Unit 7 Practice Questions
Question
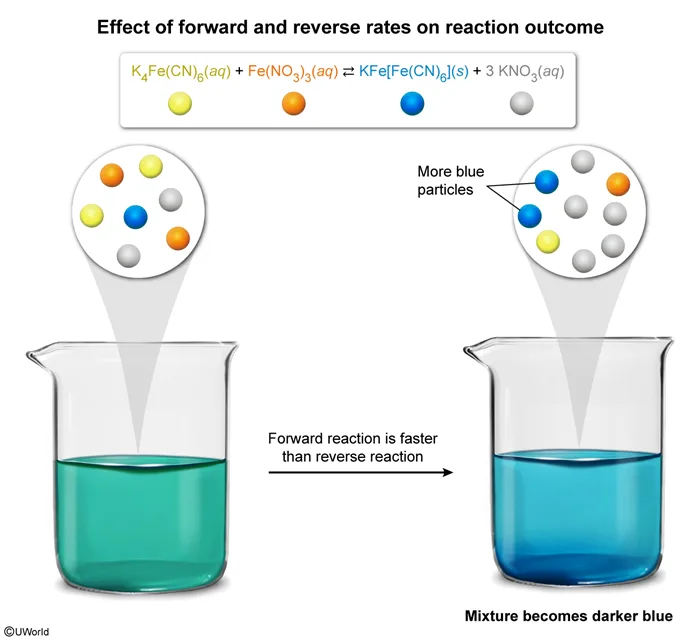
K4Fe(CN)6(aq) + Fe(NO3)3(aq) ⇄ KFe[Fe(CN)6](s) + 3 KNO3(aq)
K4Fe(CN)6(aq) and Fe(NO3)3(aq) are yellow solutions. KFe[Fe(CN)6](s) is a blue solid that can be suspended in water, and KNO3(aq) is a colorless solution. If these four compounds are mixed together so that the rate of the forward reaction is twice as fast as the reverse reaction, then
| A. the amount of KNO3 will increase, resulting in a mixture with less color. | |
| B. the amount of KFe[Fe(CN)6] will increase, resulting in a darker blue suspension. | |
| C. the amounts of K4Fe(CN)6 and Fe(NO3)3 will increase, giving the mixture a yellower appearance. | |
| D. product and reactant levels will remain constant because the mixture contains twice as many product molecules as reactant molecules. |
Explanation
In reversible reactions, reactants can be converted to products and products can be converted back to reactants. When products and reactants are present in the same vessel, both the forward and reverse reactions occur simultaneously. If the forward reaction is faster than the reverse reaction, there will be a net formation of products. If the reverse reaction is faster, there will be a net formation of reactants. If forward and reverse reactions occur at the same rate, the system is in equilibrium, and no net formation of products or reactants occurs.
The question states that the given forward reaction is twice as fast as the reverse reaction, and therefore the products (KFe[Fe(CN)6] and KNO3) will form faster than the reactants. KNO3(aq) is colorless and will not contribute to the appearance of the final mixture, but KFe[Fe(CN)6](s) is blue. As more KFe[Fe(CN)6](s) forms, the suspension will become a darker blue.
(Choice A) Although more KNO3 forms, it is colorless and does not contribute to the appearance of the final mixture.
(Choice C) K4Fe(CN)6 and Fe(NO3)3 are reactants and are consumed by the forward reaction. Because the forward reaction is faster than the reverse reaction, the amounts of these reactants decrease.
(Choice D) The question says that the forward reaction is faster than the reverse reaction (ie, net product formation occurs). It does not give any information about actual product or reactant amounts.
Things to remember:
In a reversible process, products form when the forward reaction is faster than the reverse reaction, reactants form when the reverse reaction is faster, and no change occurs when the forward and reverse reactions proceed at the same rate.
Question
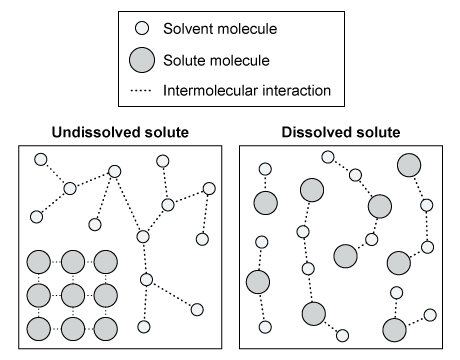
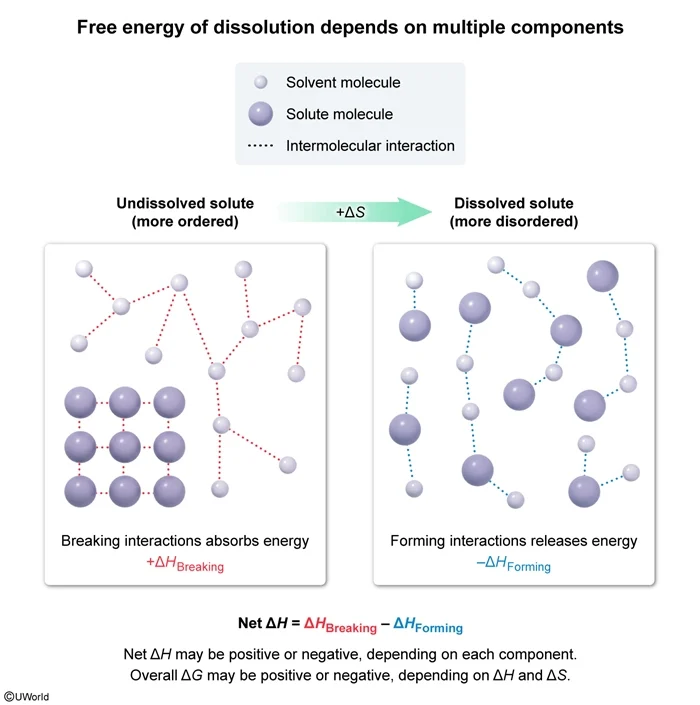
The diagram above shows the interactions between the solute and solvent molecules of a sample in both the dissolved and undissolved states. Based on the diagram, which of the following statements is true concerning the sign of the overall change in Gibbs free energy (ΔG), the net change in enthalpy (ΔH), and the change in entropy (ΔS) for the solute dissolution?
| A. ΔG is positive, because the net ΔH is negative and ΔS is positive. | |
| B. ΔG is negative, because the net ΔH is negative and ΔS is positive. | |
| C. ΔG is negative, because the net ΔH is positive and ΔS is positive. | |
| D. ΔS is positive, but the signs of ΔG and ΔH cannot be determined without additional information. |
Explanation
The Gibbs free energy change (∆G) of a process indicates the energy requirements of that process. A process with a negative ∆G can proceed without energy input, whereas a process with a positive ∆G requires energy input to occur. The value of ∆G depends on the enthalpy change (∆H), the entropy change (∆S), and the temperature T of the system as described by the equation
∆G = ∆H − T∆S
Dissolution of a solute often results in greater disorder and increased entropy (ie, a positive ∆S). The net enthalpy of dissolution is the sum of the enthalpy changes caused by breaking the initial solute-solute and solvent-solvent interactions (positive ∆H contributions) and forming new solute-solvent interactions (negative ∆H contribution).
∆H = ∆HBreaking − ∆HForming
If the negative enthalpy contributions exceed the positive enthalpy contributions, the net ∆H is negative. Otherwise, the net ∆H is zero or positive.
The diagrams show increased disorder in the dissolved state, indicating a positive ∆S. The sign of ∆G depends on whether ∆H is positive or negative, and whether ∆H can overcome any contributions from T∆S. The diagrams do not give any information about the types or relative strengths of the intermolecular interactions shown, and therefore neither the sign of ∆H nor of ∆G can be determined from the given information.
Things to remember:
The free energy ∆G of dissolution depends on the change in entropy ∆S and on the net enthalpy change ∆H that results from both the breaking and forming of intermolecular forces.
Question
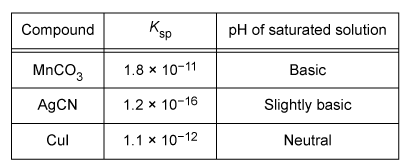
Adding an acid would increase the solubilities of which of the compounds listed in the table?
| A. CuI only | |
| B. MnCO3 only | |
| C. MnCO3 and AgCN only | |
| D. MnCO3, AgCN, and CuI |
Explanation
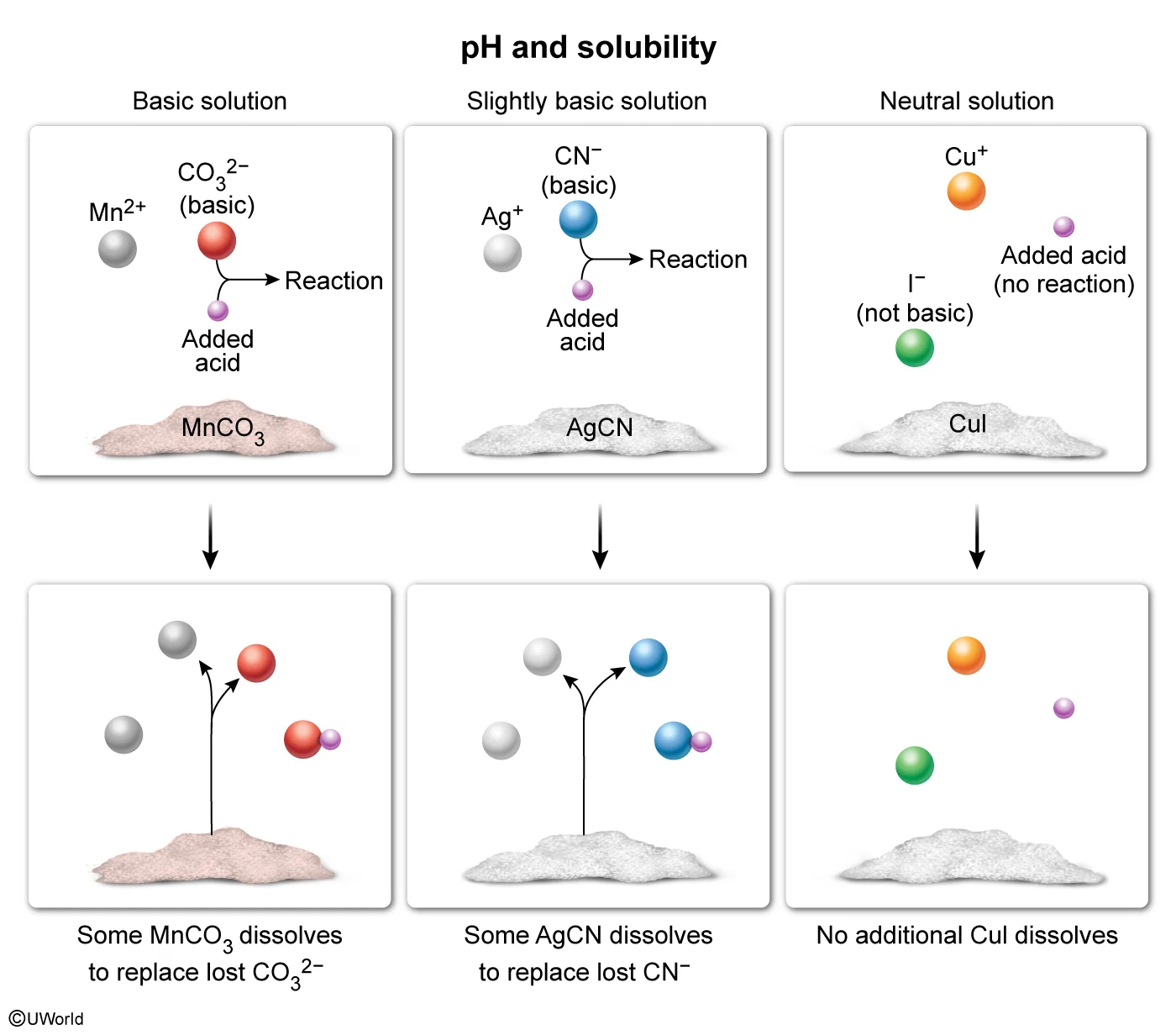
The solubility of some ionic compounds depends on the acidity or basicity of the solution. If either ion in the compound is a base, it can react with added acid (and vice versa). This acid-base reaction decreases the concentration of the reacting ion. Based on Le Châtelier's principle, this causes additional solid to dissolve and replace the ions that reacted.
According to the table, saturated solutions of MnCO3 and AgCN are at least slightly basic, indicating that one of the ions in each compound is a base (specifically, CO32− and CN− are bases). When acid is added, CO32− and CN− can react with the acid, allowing more of each compound to dissolve. In contrast, CuI produces a solution with neutral pH, indicating that neither ion is acidic or basic. As such, Cu+ and I− will not react with added acid and adding acid will not affect the solubility of CuI. Therefore, only MnCO3 and AgCN would increase in solubility upon addition of an acid.
Things to remember:
The solubility of a salt in which one ion is an acid or base depends on the pH of the solution. Adding an acid to a salt with a basic ion increases its solubility and adding a base decreases its solubility. The opposite is true for salts with an acidic ion.
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chemistry Unit 7 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 7: Equilibrium?
AP Chemistry Unit 7 focuses on chemical equilibrium and the dynamic balance in chemical reactions. Key topics include:
- Introduction to equilibrium
- Calculating the equilibrium constant
- Calculating equilibrium concentrations
- Introduction to Le Châtelier’s principle
- Introduction to solubility equilibria
How should I prepare for an AP Chemistry Unit 7 exam?
Start by reviewing your class notes and textbook examples to grasp the main concepts. Then you can do the following:
- Watch short, focused video lessons for visual learning.
- Complete practice questions and timed quizzes, including AP Chemistry Unit 7 MCQ, to mimic test conditions.
- Focus on important formulas like K, Q, and Ksp calculations.
- Use flashcards for key terms and principles, such as Le Châtelier’s Principle
- Consistent, active practice instead of memorization is the key to mastering Unit 7.
Are any free resources available for AP Chemistry Unit 7?
Yes! There are several ways to access free AP Chemistry content online:
- College Board’s AP Classroom provides sample questions and unit guides.
- Khan Academy has free tutorials covering equilibrium and buffer systems.
While free resources are great for extra learning, think about combining them with structured practice from UWorld to make sure you’re ready for the exam. Improve your prep by using both free and premium resources!
What types of questions are on the AP Chemistry Unit 7 test?
The Unit 7 questions can appear in different formats:
- Multiple-choice questions (MCQs): These often involve calculating equilibrium constants, comparing Q and K, or predicting reaction shifts.
- Free-response questions (FRQs): These require explanations of Le Châtelier’s Principle, calculations, or analyses of experimental data.
What is the weight of AP Chemistry Unit 7 on the exam?
Equilibrium topics account for about 7 to 9% of the AP Chemistry exam. While it isn’t the largest unit, it is high-yield because questions frequently combine several concepts, such as kinetics and thermodynamics. A solid understanding can increase your confidence in both MCQs and FRQs.
What are the common mistakes students make in AP Chem Unit 7?
Students often stumble on:
- Confusing Q and K values when predicting reaction shifts.
- Forgetting to account for temperature changes in Le Châtelier’s Principle.
- Miscalculating solubility or equilibrium constants.
- Ignoring the effect of catalysts, which do not change the equilibrium, is important for understanding this concept.
Avoid these errors by practicing problems carefully and double-checking calculations. A little extra care can save points on the AP exam!
How do I self-study for AP Chemistry Unit 7 effectively?
Effective self-study combines active learning and regular review.
- Create a study schedule that breaks down topics like K calculations, buffers, and solubility.
- Solve practice questions right after reviewing each concept.
- Teach the concepts out loud or explain them to a peer; it helps reinforce understanding.
Track your mistakes and consistently revisit areas where you struggle. Self-study is all about consistency. Make it interactive, not passive, to improve retention!
How can I improve my score on the Free-Response Questions (FRQs) for Unit 7?
To excel on AP Chem Unit 7 FRQs:
- Practice step-by-step solutions; always show your work for calculations.
- Label units and significant figures carefully.
- Use diagrams to support explanations when you can.
- Review previous years’ FRQs to understand common patterns.
Practice under timed conditions to mimic the real exam. Start working on FRQs now to turn tricky questions into easy points!
Where can I find a good study guide for AP Chemistry Unit 7?
Several options exist:
- UWorld’s AP Chemistry platform offers interactive study guides with practice questions.
- AP Classroom (College Board) gives free unit-specific resources and progress checks.
Pairing a structured guide with active problem-solving is the fastest way to master Unit 7. Dive in and see your understanding improve!
Can I find practice tests specifically for AP Chem Unit 7?
Yes! Many platforms provide practice focused on Unit 7:
- UWorld offers targeted practice questions and mini-quizzes for equilibrium.
- Khan Academy and College Board AP Classroom include topic-specific questions.
- Some review books also provide chapter-based practice tests.
Use these tests to find weak areas and review important concepts. Practicing now can give you a big advantage on exam day!
What are the high-yield Unit 7 topics most frequently tested on the AP Chemistry exam?
Focus on these important topics:
- Le Châtelier’s Principle and predicting how reactions shift.
- Calculating equilibrium constants (Kc, Kp).
- Solubility product (Ksp) and the common ion effect.
- Buffer calculations and the Henderson-Hasselbalch equation.
- Comparing Q and K to determine the direction of reactions.
Mastering these will get you ready for both multiple-choice questions and free-response questions. Don’t overlook the tricky calculations; they often earn you points!
What is the best way to review AP Chemistry Unit 7 before the AP exam?
The most effective review combines concept refreshers with active practice.
- Re-watch concise video lessons to reinforce key ideas.
- Solve a variety of practice problems, from multiple-choice questions to free-response questions.
- Use interactive study guides to test formulas, graphs, and reactions.
- Focus on important and frequently tested topics for maximum efficiency.
End your review by simulating exam conditions with timed questions. Start today and make Unit 7 one of your strongest sections!