AP® Chemistry Unit 6 Review and Practice Test
Master the core concepts of AP® Chemistry Unit 6: Thermochemistry with our in-depth review. This section explores energy changes in chemical reactions, including heat transfer, enthalpy, calorimetry, and Hess’s Law, the foundation of chemical energetics. Strengthen your understanding through engaging lessons, exam-style multiple choice questions (MCQs), and full-length practice tests that mirror the AP Chemistry exam. Whether you prefer quick notes, visual explanations, or in-depth problem sets, this AP Chem Unit 6 review helps you master the science of energy and build confidence for test day.
Boost Your Confidence and Score High with Our AP Chemistry Unit 6 Review
Explore AP Chemistry Unit 6: Thermochemistry with a complete set of tools designed to help you succeed. This AP Chemistry Unit 6 review covers energy flow, enthalpy changes, calorimetry, and thermochemical equations. Practice with AP Chemistry Unit 6 MCQs and practice tests that mirror the real exam, and reinforce your learning with our step-by-step study guide and engaging video lessons.
Engaging Video Lessons
Challenging topics like enthalpy, calorimetry, and Hess’s Law are simplified through clear, visual explanations. Learn faster with short, high-yield AP Chemistry Thermochemistry video lessons designed specifically for AP Chem students preparing for the Thermochemistry unit.
Interactive Study Guides
Our AP Chemistry Thermochemistry study guide follows every topic outlined in the College Board’s CED. Built-in knowledge checks and quick AP Chemistry Unit 6 notes help you master energy flow, enthalpy changes, and calorimetry before your test.
Try These AP Chemistry Unit 6 Practice Test Questions
Question
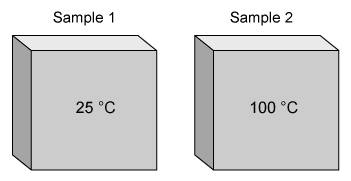
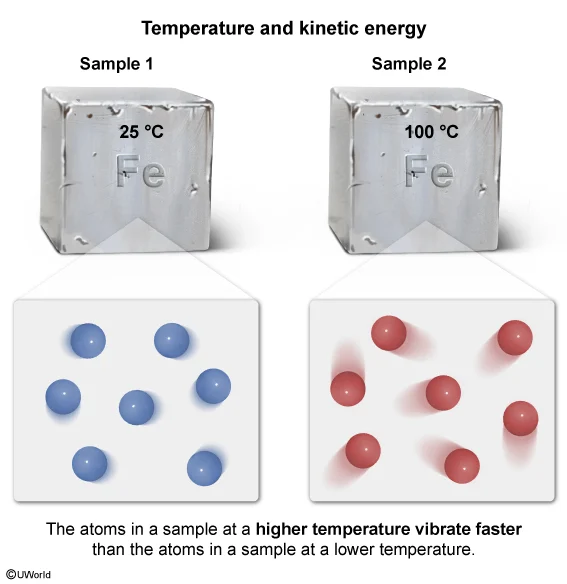
The figure above represents two samples of iron, each at different temperatures. In which sample are the vibrations of the atoms the fastest?
| A. Sample 1 | |
| B. Sample 2 | |
| C. The atoms in both samples have the same kinetic energy. | |
| D. It cannot be determined without knowing the mass of the samples. |
Explanation
At all temperatures above absolute zero, the atoms and molecules in matter are in constant motion. In solids, the motion is primarily vibrational (ie, atoms vibrating in one place). The kinetic energy (energy of motion) of the atoms and molecules in a sample is proportional to the temperature of the sample. Therefore, as the temperature of the sample increases, the kinetic energy of the atoms and molecules in the sample increases.
For the two samples of iron described in this question, sample 1 is at a lower temperature than sample 2. Because atoms vibrate faster at higher temperatures, the atoms in sample 2 are vibrating faster than the atoms in sample 1.
(Choice A) Because kinetic energy is proportional to temperature, the atoms in sample 1 (lower temperature sample) are vibrating slower than the atoms in sample 2.
(Choice C) Because temperature is proportional to kinetic energy and the samples are at different temperatures, the atoms in sample 1 and sample 2 cannot have the same kinetic energy.
(Choice D) The mass of an object does not affect the rate of vibration of its individual particles. The masses of the individual particles affect the vibrational rate but because both samples are the same substance (iron, in this case), the atoms in each have the same average mass.
Things to remember:
The kinetic energy of the atoms and molecules in a sample is proportional to the temperature of the sample. As the temperature of a sample increases, the kinetic energy of the atoms and molecules in the sample increases.
Question
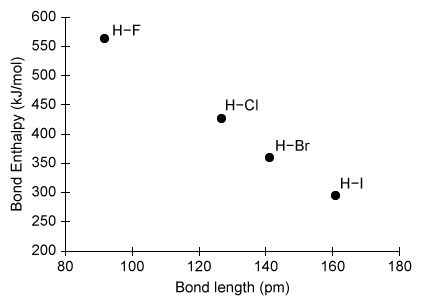
The strength of a chemical bond can be measured using bond enthalpies. Based on the bond enthalpies shown in the graph above, which of the following radical reactions will be most exothermic?
| A. HCl(g) + CH3(g) → Cl(g) + CH4(g) | |
| B. HBr(g) + CH3(g) → Br(g) + CH4(g) | |
| C. HF(g) + CH3(g) → F(g) + CH4(g) | |
| D. HI(g) + CH3(g) → l(g) + CH4(g) |
Explanation
Bond enthalpy (ΔH°bond), also known as bond dissociation energy, is the amount of energy needed to break a particular bond in 1 mole of a molecule in the gas phase. The amount of energy associated with a bond indicates its strength (eg, stronger bonds require more energy to break than weaker bonds). During a chemical reaction, existing bonds must absorb energy to break (an endothermic process with +ΔH°bond), and when new bonds are formed, energy is released (an exothermic process with −ΔH°bond).
The ΔH° of a chemical reaction can be calculated using sums of measured bond enthalpies, according to the following equation:
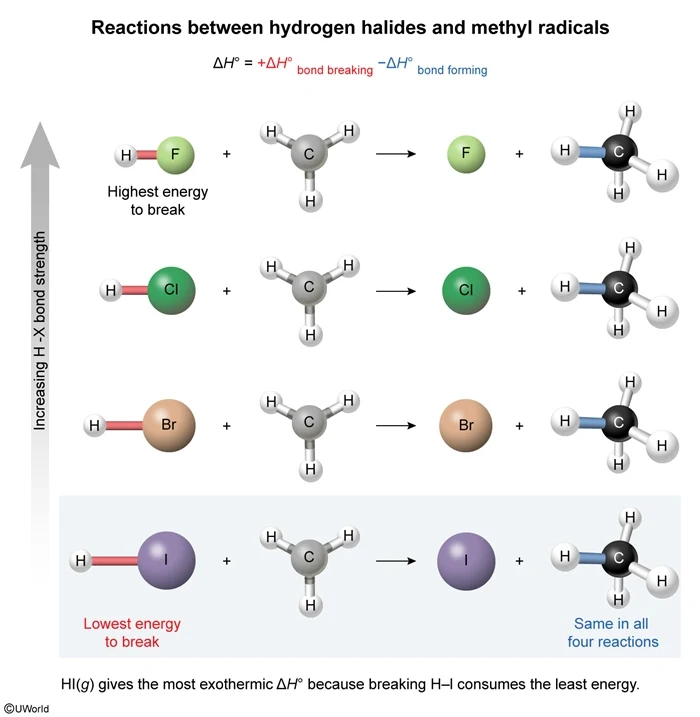
In this question, hydrogen halides (H–X, where X = F, Cl, Br, and I) are reacted with methyl radicals (CH3) to form halogen atoms (X) and methane (CH4). The amount of energy released from bond formation is the same in all four reactions because each reaction forms one C–H bond. As such, the net ΔH° of each reaction depends on how much energy is consumed in breaking the H–X bond. According to the graph above, the H-I bond has the lowest ΔH°bond and therefore consumes the least energy when broken. As a result, the reaction between H–I and CH3 will be most exothermic.
(Choices A and B) Both H–Cl and H–Br have bond enthalpies greater than that of H–I; therefore, their reactions with CH3 will release less energy.
(Choice C) H–F has the largest bond enthalpy (ie, absorbs the most energy when broken); consequently, the reaction between H–F and CH3 will be least exothermic.
Things to remember:
The energy required to break a chemical bond is called the bond enthalpy (ΔH°bond). Breaking bonds absorbs energy (+ΔH°bond), whereas forming bonds releases energy (−ΔH°bond). The net enthalpy of a reaction (ΔH°) is the sum of ΔH°bond from each bond-breaking and bond-forming step.
Question
NH4NO3(s) → NH4+(aq) + NO3−(aq)
A student dissolves NH4NO3(s) into H2O(l) and observes an endothermic reaction that proceeds according to the net-ionic equation above. Which of the diagrams below best represents the heat transfer and change in solution temperature T observed by the student during the reaction?
A. 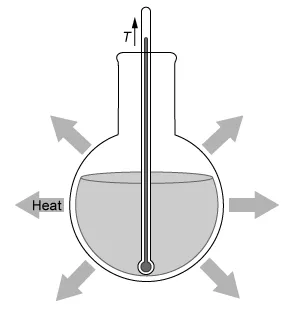 |
|
B. 
|
|
C. 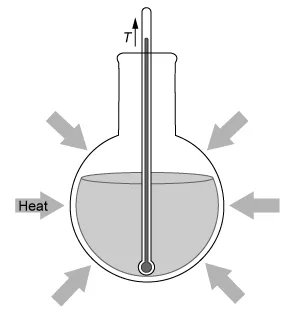 |
|
D. 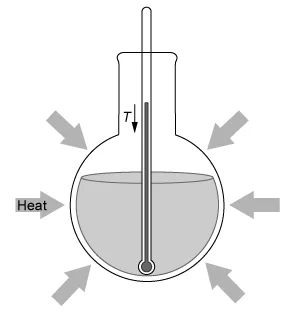 |
Explanation
An exothermic reaction is a process that releases energy (eg, heat, light) to the surroundings.
Reactants → Products + Energy
Because energy is liberated as a product of an exothermic reaction, the reaction mixture's temperature increases as heat is generated and then transferred from the warmer reaction system to the cooler surrounding environment.
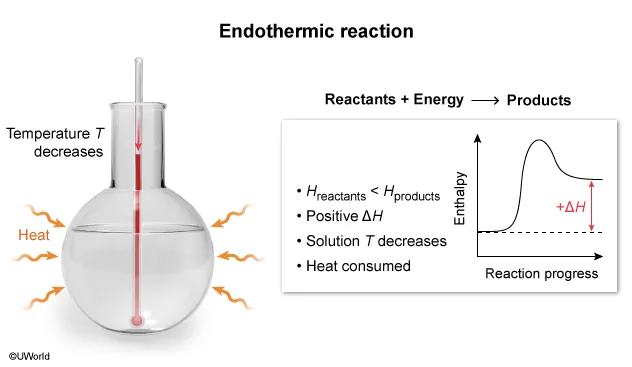
Conversely, an endothermic reaction is a process that absorbs energy from the surroundings.
Reactants + Energy → Products
Because energy is consumed like a reactant in an endothermic reaction to enable the reaction to proceed, the reaction mixture's temperature decreases, and heat is absorbed from the warmer surrounding environment by the cooler reaction system.
The change in enthalpy ΔH evaluates the change in the total energy of the system following a reaction or process.
ΔH = Hproducts − Hreactants
If the products are lower in energy than the reactants, ΔH is negative (ie, energy is released from the system). When the products are higher in energy than the reactants, ΔH is positive (ie, energy is absorbed by the system).
In this question, dissolving NH4NO3(s) into H2O(l) is observed to be an endothermic reaction. Therefore, the temperature of the reaction mixture decreases, and heat is absorbed from the warmer surrounding environment, as depicted by the diagram in Choice D.
(Choice A) This diagram depicts an exothermic reaction, but the given reaction is endothermic.
(Choice B) This diagram shows heat being generated and released from the system (an exothermic reaction) with an incorrect change in temperature. If heat is generated by the solution, the solution temperature must increase.
(Choice C) This diagram correctly shows heat being absorbed by the system (an endothermic reaction) but has an incorrect change in temperature. If heat is consumed by the reaction, the solution temperature must decrease.
Things to remember:
An exothermic reaction mixture's temperature increases as heat is generated and then released from the system to the surroundings. An endothermic reaction mixture's temperature decreases as heat is absorbed from the surroundings and consumed by the reaction system.

Study Anywhere, Anytime
Tackle practice questions during your commute, review quick video lessons between classes, or brush up on thermochemistry concepts while relaxing at your favorite café. With the UWorld app, your complete AP Chemistry Unit 6 review and Thermochemistry practice tests are always just a tap away.
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chemistry Unit 6 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 6: Thermochemistry?
Unit 6 focuses on how energy changes occur during chemical and physical processes. Key concepts include:
- Heat flow and energy transfer: Understanding how systems gain or lose energy.
- Enthalpy (ΔH): Measuring energy changes in reactions.
- Calorimetry: Using experimental data to calculate heat exchange.
- Bond energy and reaction energetics: Exploring how bond formation and breaking relate to energy.
- Hess’s Law: Predicting reaction enthalpies using known data.
These AP Chemistry Thermochemistry topics explain why some reactions release heat while others absorb it, forming the basis for kinetics and equilibrium.
How is “Thermochemistry” tested and weighted on the AP Chemistry exam?
How should I prepare for an AP Chemistry Unit 6 exam?
Begin by reviewing key terms, formulas, and concepts like heat flow and enthalpy to build a strong foundation. Practice calorimetry and Hess’s Law calculations to reinforce quantitative understanding. Use visuals or diagrams to connect equations with real-life chemical systems.
As the test approaches, take timed practice sets to improve accuracy and pacing, an essential part of AP Chemistry Unit 6 test prep. UWorld’s question bank is a great way to simulate the real AP experience while tracking your progress.
What’s the best way to self-study for Unit 6 AP Chemistry?
What are some common errors students make in AP Chem Unit 6?
How can I score higher on the Free-Response Questions (FRQs) for Unit 6?
Are any free resources available for AP Chemistry Unit 6?
Where can I get a reliable study guide for AP Chemistry Unit 6?
The UWorld AP Chemistry study guide is one of the most reliable resources for mastering Unit 6: Thermochemistry. It provides comprehensive coverage of energy changes, enthalpy, calorimetry, and Hess’s Law, all fully aligned with the College Board’s CED.
Each section of the guide includes concise explanations, clear visuals, and step-by-step worked examples that make complex thermodynamic concepts easy to understand. You’ll also find practice questions and “Check for Understanding” exercises to reinforce learning and apply key formulas accurately.
Can I access practice tests that focus only on AP Chem Unit 6?
Yes! UWorld lets you generate custom quizzes and full-length AP Chemistry Unit 6 progress check MCQs focused solely on Thermochemistry topics. You can target specific concepts like calorimetry, enthalpy, and Hess’s Law for efficient, goal-oriented review. Each question includes visual explanations, detailed step-by-step solutions, and answer rationales to strengthen your conceptual understanding.
When paired with UWorld’s AP Chemistry QBank, which offers thousands of exam-style questions with detailed rationales, it becomes an all-in-one solution for Unit 6 preparation, helping you recognize common mistakes and build problem-solving confidence before test day.