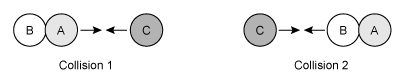AP® Chemistry Unit 5 Review and Practice Test
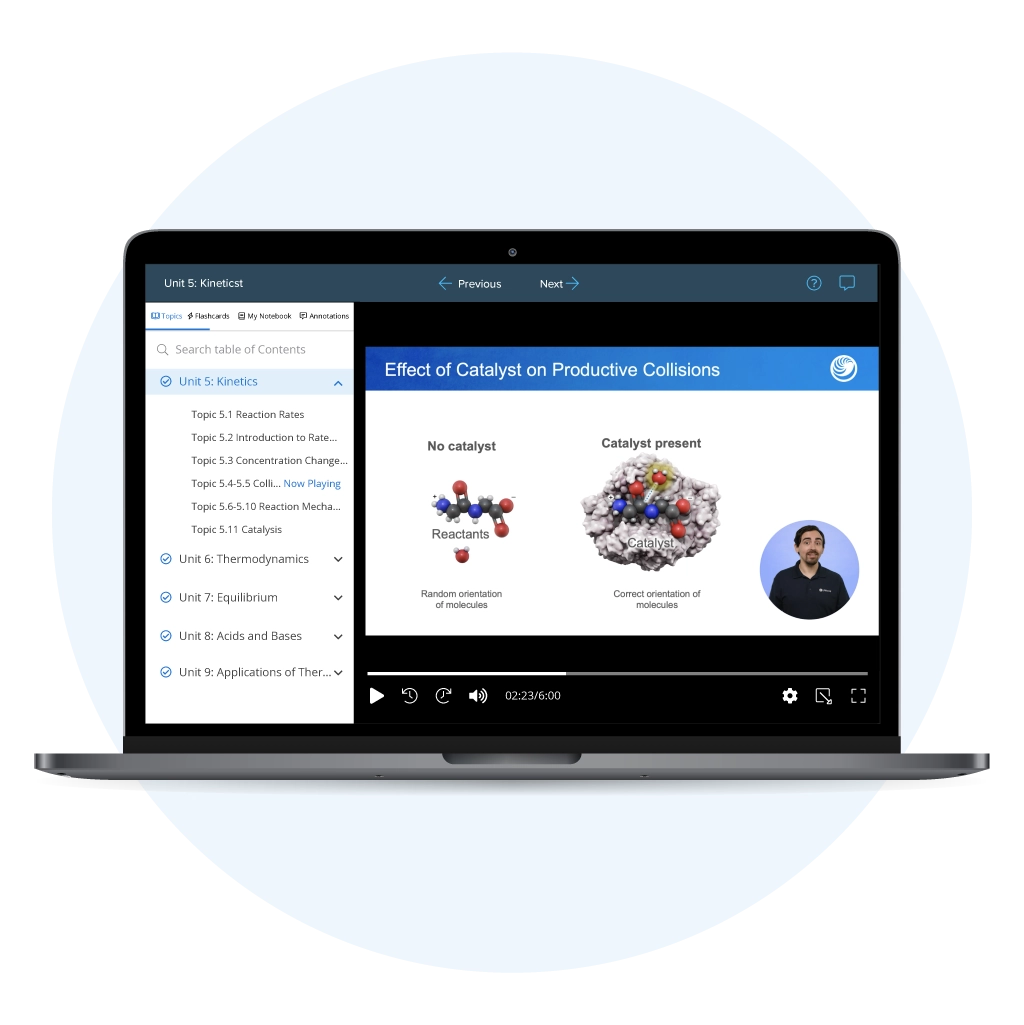
UWorld’s AP® Chemistry Unit 5 Review: Kinetics makes the most challenging topics simple and easy to master. Our AP specialists break down key concepts such as Rate Laws, Reaction Mechanisms, Catalysts, and Factors Affecting Reaction Rates with clear explanations and visuals. After learning the concepts, you can apply your knowledge through AP Chem Unit 5 MCQs and FRQs, helping you build confidence and score a solid 5 on the exam.
Boost Your Confidence and Score High with Our AP Chemistry Unit 5 Review
Take the stress out of AP® Chemistry Unit 5 prep with our comprehensive review on chemical kinetics. Our expert-designed resources, including video lessons, practice questions, and detailed study tools, guiding you step by step through tough topics and helping you learn efficiently to perform your best on test day.
Engaging Video Lessons
Don’t let heavy textbooks slow you down! UWorld’s short, engaging video lessons make studying for AP Chemistry Chemical Kinetics simple and effective. Each lesson is designed to help you understand faster, stay focused, and retain longer.
Interactive Study Guides
Designed according to the College Board® CED, our interactive study guide provides the most accurate and up-to-date content. Each AP Chem Unit 5 topic includes structured notes, labeled illustrations, and built-in comprehension checks to reinforce learning thoroughly.
Try These AP Chemistry Unit 5 Practice Test Questions
Question
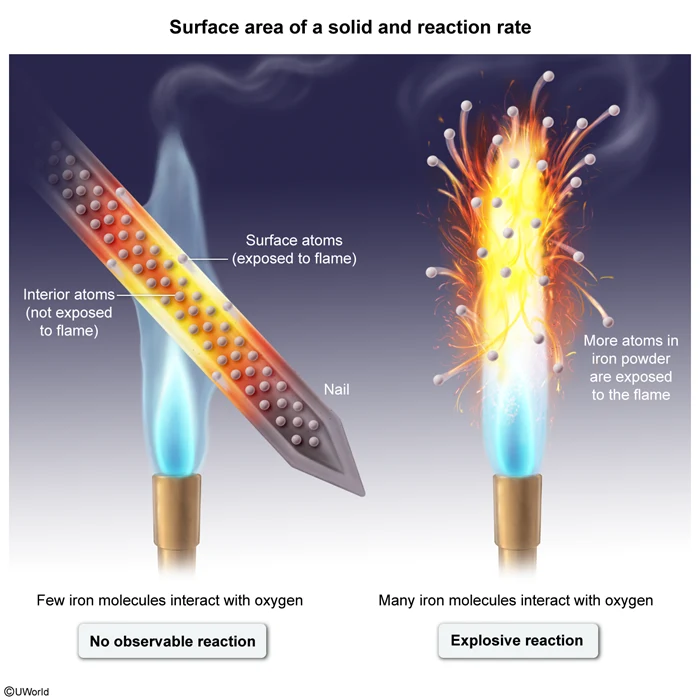
When a clean iron nail with a mass of 20. g is exposed to a flame, no reaction happens. When a 20. g sample of iron filings ground into a fine powder is exposed to a flame, it reacts explosively according to the equation above. Which of the following statements correctly explains why the second reaction occurs but not the first?
| A. The surface area of the iron filings is greater than the surface area of the nail. | |
| B. The boiling point of the filings is lower than the melting point of the nail. | |
| C. The intermolecular forces in the iron filings are weaker than those in the nail. | |
| D. The oxidation number of the iron atoms in the filings is greater than that of the atoms in the nail. |
Explanation
The rate of a chemical reaction depends on the frequency of collisions between particles. Faster reactions have more frequent collisions than slower reactions. Molecular collisions can occur more frequently when more particles are exposed to each other. For solids, an increased surface area leads to more frequent collisions between the solid and its surroundings.
The question asks about two samples of iron of equal mass (ie, equal number of particles in each). In the first sample, most of the iron atoms are on the inside of the nail (ie, little surface area), so these atoms do not interact with the surrounding air. This causes the reaction to happen so slowly that it effectively does not occur at all.
In contrast, the second sample is a fine powder. More of the iron atoms in this sample are exposed to the surrounding air (ie, the powder has a greater surface area than the nail), allowing more frequent collisions and a much faster, explosive reaction.
(Choices B, C, and D) Both samples are made of elemental iron and as such have the same melting point, intermolecular forces, and oxidation number. Additionally, these properties do not impact the rate of a reaction.
Things to remember:
The rate of a reaction increases with increasing frequency of collisions between particles. For solids, collisions with surrounding gas or liquid particles are more frequent when the solid has a greater surface area.
Question
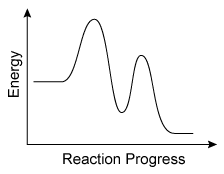
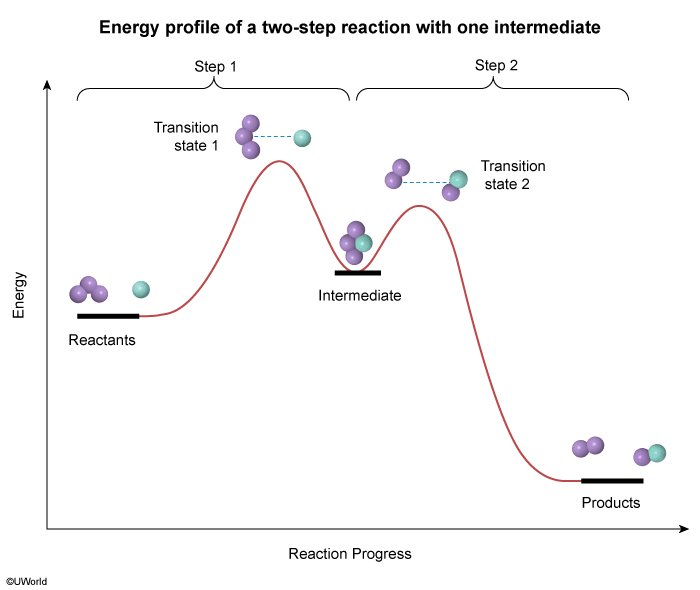
The energy profile of a reaction is shown in the diagram above. Based on the profile, which of the following correctly states the number of transition states involved in the reaction and the number of chemical intermediates that are formed during the reaction?
| Transition States | Intermediates | ||
| A. 1 | 2 | ||
| B. 2 | 1 | ||
| C. 2 | 2 | ||
| D. 3 | 1 |
Explanation
During a reaction, existing bonds are broken, and new bonds are formed. Each step in this process results in a momentary, high-energy transition state that is seen as a peak on a reaction energy diagram (one peak for each step). Intermediates that form during the process appear as "valleys" between the peaks.
For the reaction in this question, the energy profile in the diagram has two peaks, indicating that the reaction happens in two steps and forms two transition states (one for each step). The energy profile also shows one valley between the peaks, indicating that one intermediate is formed.
Therefore, there are two transition states and one chemical intermediate formed during the reaction.
(Choices A and C) The two peaks in the energy profile indicate that there are two steps in the reaction, but these peaks signify transition states, not intermediates.
(Choice D) There are a total of three arcs in the profile (ie, two "peaks" and one "valley"), but only the peaks indicate the number of transition states. The "valley" between the peaks indicates an intermediate.
Things to remember:
On a reaction energy diagram, one transition state peak is seen for each elementary step in the reaction mechanism. Intermediates that form during the reaction process appear as "valleys" between the peaks.
Question
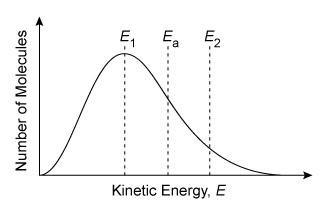
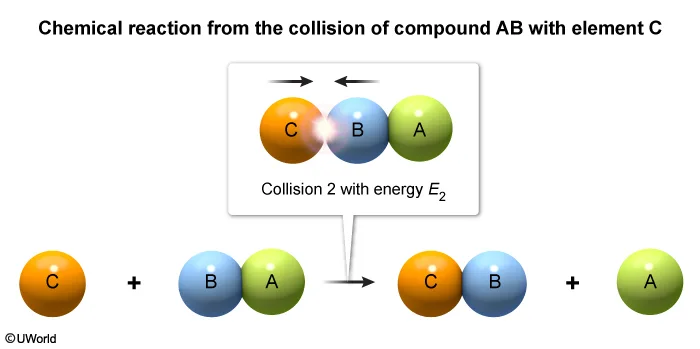
Consider the single-step reaction AB + C → BC + A, in which one particle of compound AB collides with a particle of element C. Two possible collision orientations are shown above, and the kinetic energy distribution of the reacting molecules and the activation energy Ea of the reaction are shown in the graph below.
Which of the following collision orientations and energies (E1 or E2) is most likely to be effective in forming the products BC and A?
| A. Collision 1 with energy E1 | |
| B. Collision 1 with energy E2 | |
| C. Collision 2 with energy E1 | |
| D. Collision 2 with energy E2 |
Explanation
According to collision theory, a reaction can occur between two molecules only when the molecules collide with enough energy and the correct orientation. The minimum energy needed for the reactant molecules to reach the transition state and initiate a reaction is called the activation energy Ea. Molecules that collide with less than the minimum Ea cannot react to form products.
At a given temperature, some molecules of a liquid or a gas in a reaction vessel move more slowly (lower kinetic energy) while other molecules move more quickly (higher kinetic energy). This causes the molecules to display a wide range of kinetic energies, which is represented graphically by the Maxwell-Boltzmann distribution.
The area under a portion of the Maxwell-Boltzmann distribution curve indicates the percentage of the total number molecules that exist within that range of kinetic energies (more area means a greater percentage). Increasing the temperature shifts and widens the distribution toward higher molecular speeds (ie, higher kinetic energies). However, at any temperature, the only molecules that can react are those with an energy that is greater than or equal to Ea.
Two molecules with enough energy to react must also be oriented so that new bonds can be formed between two atoms as other bonds are broken. In this question, only collision 2 orients the molecules so that atoms B and C can interact to form a new B–C bond as the A–B bond is broken.
(Choices A and B) These scenarios will not form products because they have an incorrect collision orientation.
(Choice C) This scenario has the correct collision orientation but will not form products because it has insufficient energy (E1 < Ea).
Things to remember:
A reaction can occur between two molecules only when the molecules collide in the correct orientation and with an energy that is greater than or equal to the activation energy for the reaction.

Study Anywhere, Anytime
Tackle AP Chem unit 5 practice questions on the ride home from a game, review topic videos between classes, or dive into a study guide while waiting for friends at the coffee shop. Keep everything you need to ace the AP Chemistry unit 5 exam right at your fingertips. Get the app today!
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chemistry Unit 5 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 5: Kinetics?
Following are the topics covered in AP Chem Unit 5: Kinetics:
- Reaction Rates
- Introduction to Rate Law
- Concentration Changes Over Time
- Elementary Reactions
- Collision Model
- Reaction Energy Profile
- Introduction to Reaction Mechanisms
- Reaction Mechanism and Rate Law
- Pre-Equilibrium Approximation
- Multistep Reaction Energy Profile
- Catalysis
How should I prepare for an AP Chemistry Unit 5 exam?
Preparing effectively for the Unit 5 AP Chemistry exam takes planning, consistency, and the right study tools.
- Start your preparation 2–3 months in advance to give yourself enough time to review all major topics.
- Create a practical study schedule that you can stick to without feeling overwhelmed.
- Focus on key Kinetics concepts such as Rate Laws, Reaction Mechanisms, and Catalysts.
- Use UWorld’s AP Chemistry resources, including a prep book, question bank, flashcards, digital notebooks, and performance dashboard, for guided, exam-focused learning.
- Track your progress regularly and revisit difficult concepts to strengthen understanding before test day.
Are any free resources available for AP Chemistry Unit 5?
Yes! UWorld offers a 7-day free trial that gives you a firsthand experience of our AP Chemistry Unit 5 study materials. During the trial, you can explore exam-level questions, in-depth video lessons, and expert explanations that simplify even the toughest Kinetics concepts. Once you’ve experienced the quality of our content, you can upgrade to the full course for complete access to all AP chemistry Unit 5 questions, prep book, study tools, and performance tracking features.
What types of questions are on the AP Chem Unit 5 test?
The AP Chemistry Unit 5: Kinetics exam includes two main question types: Multiple-Choice Questions (MCQs) and Free-Response Questions (FRQs), each contributing equally to your final score. The MCQs assess your understanding of core concepts like rate laws, reaction mechanisms, and catalysts, while FRQs challenge you to apply these ideas in experimental and data-based scenarios.
What are common mistakes students make in AP Chem Unit 5?
Many students struggle with AP Chemistry Unit 5: Kinetics because they miss crucial details or rely solely on memorization. Here are some frequent pitfalls:
- Misinterpreting rate laws or overlooking how concentrations affect reaction rates.
- Confusing reaction mechanisms, which can lead to errors in predictions or calculations.
- Neglecting units or skipping steps in problem-solving, affecting accuracy.
- Relying only on memorization without understanding the underlying concepts.
Avoid these mistakes and build a stronger foundation with UWorld’s AP Chemistry course. With expertly designed video lessons, practice questions, study guides, and progress tracking, you’ll master every concept, gain confidence, and be fully prepared for exam day.
How do I self-study for AP Chemistry Unit 5 effectively?
How can I improve my score on the Free-Response Questions (FRQs) for Unit 5?
What is the "Kinetics" unit's weight on the AP Chemistry exam?
Where can I find a good study guide for AP Chemistry Unit 5?
Most students turn to UWorld’s AP Chemistry Unit 5 study guide for its comprehensive coverage, clear explanations to AP chemistry Unit 5 progress check MCQ answers, and visually engaging illustrations. Each section includes in-depth guidance and “Check-for-Understanding” questions to help reinforce your learning. The guide is designed to simplify complex concepts and support both self-study and classroom review. You can purchase it conveniently through Amazon or directly on UWorld’s website to get started on studying Unit 5 today.
Can I find practice tests specifically for AP Chem Unit 5?
UWorld makes it easy to target your AP Chemistry Unit 5 preparation with customized practice tests designed to help you master each concept.
- Topic-wise practice: Focus on specific Kinetics topics to strengthen weak areas.
- Unlimited attempts: Practice questions as many times as you need to gain confidence.
- Exam-level difficulty: Questions mirror the style and challenge of the actual AP exam.
- Instant feedback: Detailed explanations help you understand mistakes and improve.
With UWorld’s full AP Chem course, you get access to all practice tests, video lessons, and study tools, giving you a complete, structured way to prepare and maximize your score.
What are Unit 5 topics most frequently tested on the AP Chemistry exam?
Do AP Chemistry Unit 5 topics appear again in later units?
Yes! Many concepts from AP Chemistry Unit 5: Kinetics, such as reaction rates, rate laws, and reaction mechanisms, reappear in later units and are often applied in more complex contexts. Understanding these foundational topics thoroughly is essential, as they form the basis for solving problems in thermodynamics, equilibrium, and chemical reactions in subsequent units.