AP® Chemistry Unit 4 Review and Practice Test
Prepare for AP® Chemistry Unit 4 by using our review guide. It provides clear explanations, sample questions, and FRQs to help you better understand chemical reactions.
Ace AP Chemistry Unit 4 with Smart Prep.
Increase your chances of scoring high using our AP Chemistry Unit 4 review. You can reinforce concepts of chemical reactions by practicing targeted AP Chemistry Unit 4 practice questions. Working through sample free-response questions will help you understand the problem-solving steps involved.
Engaging Video Lessons
Struggling with a tricky topic? Hit pause, rewind, or watch again; take things at your own speed. Our AP Chemistry Unit 4 video lessons make learning chemical reactions interactive and easy to follow. Each video is made to align with AP Chemistry Unit 4 practice and progress check MCQs.
Interactive Study Guides
Our AP Chemistry Unit 4 study guide breaks down key reaction concepts with clear explanations and examples. Practice effectively with our chemistry reactions quiz and progress check questions. Stay organized and focused as you prepare for the AP Chem Unit 4 test.
Challenge Yourself with AP Chemistry Unit 4 Practice Test
Question
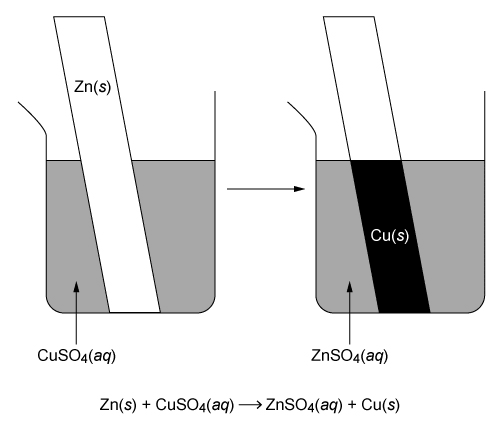
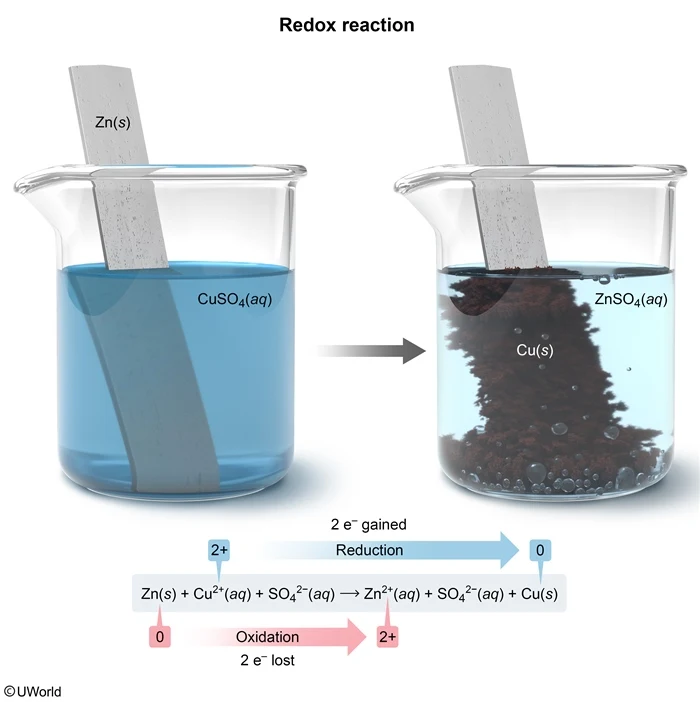
A Zn(s) strip is placed in a CuSO4(aq) solution. A redox reaction occurs, depositing a solid on the Zn(s) strip. Which of the following describes the redox reaction depicted in the diagram above?
| Species being oxidized | Species being reduced | Electrons transferred | ||
| A. Zn | SO42− | 4 | ||
| B. Cu2+ | Zn | 2 | ||
| C. Zn | Cu2+ | 2 | ||
| D. Cu2+ | SO42− | 4 |
Explanation
Redox reactions occur when one or more electrons are transferred from one atom to another. The atom that loses the electron(s) becomes oxidized, and the atom that gains the electrons from the oxidized atom becomes reduced. Oxidation and reduction must occur simultaneously in a redox reaction. The oxidation number of the oxidized atom will increase by the number of electrons lost, and the oxidation number of the reduced atom will decrease by the number of electrons gained.
This question states that a Zn(s) strip is placed in a CuSO4(aq) solution, and Cu(s) is deposited on the Zn strip as a result of the redox reaction.
Zn(s) + Cu2+(aq) + SO42−(aq) → Zn2+(aq) + SO42−(aq) + Cu(s)
Initially, the oxidation number of Zn(s) is 0 because Zn(s) is in its elemental state, but the oxidation number of the ionically bound Cu ion in CuSO4 is +2. During the reaction, Cu2+ becomes reduced to elemental Cu(s) with an oxidation number of 0.
Zn(s) → Zn2+(aq) + 2e− (oxidation)
Cu2+(aq) + 2e− → Cu(s) (reduction)
Therefore, Cu2+ gains 2 electrons, which are lost from Zn as it becomes oxidized to Zn2+. Because the oxidation numbers of Zn and Cu change by 2 units, 2 electrons are transferred in the redox reaction.
(Choices A and D) SO42− is not reduced because its oxidation number is the same on both sides of the reaction arrow, and Cu2+ is not oxidized because it gains electrons rather than losing them. In addition, 2 rather than 4 electrons are transferred in this redox reaction.
(Choice B) Zn and Cu2+ become oxidized and reduced, respectively. This choice has reversed the species being oxidized and reduced.
Things to remember:
Redox reactions occur when one or more electrons are transferred from one atom to another. An atom becomes oxidized when it loses electrons and reduced when it gains electrons. As a result of oxidation or reduction, an atom's oxidation number increases or decreases, respectively, by the number of electrons lost or gained.
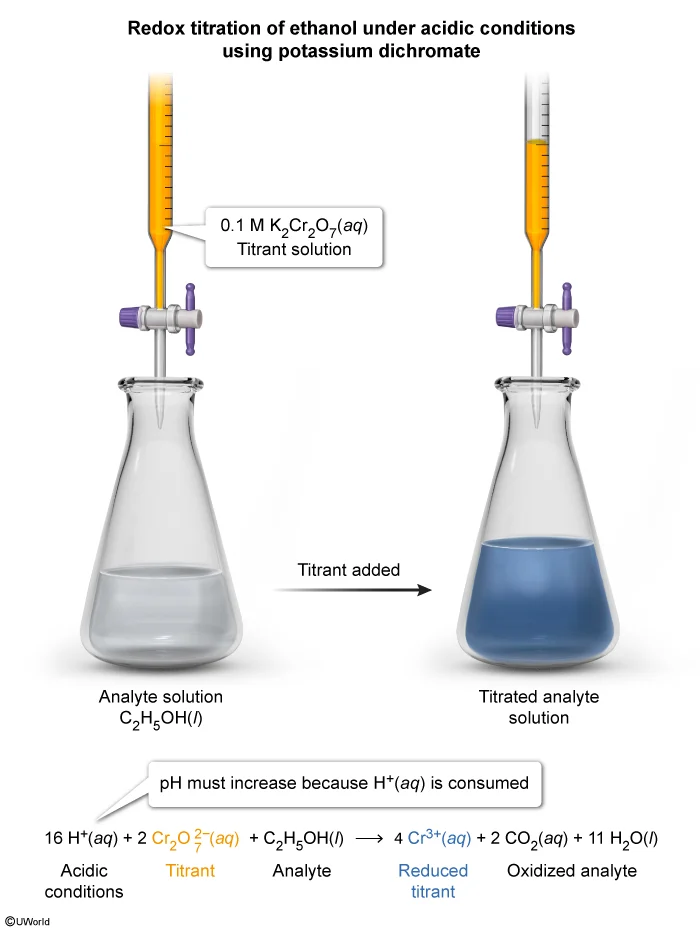
Question
An acidic solution containing an unknown amount of C2H5OH(l) is titrated with a solution of 0.1 M K2Cr2O7(aq). The endpoint is determined when the color of the unknown solution turns blue. As the titration proceeds, the pH of the solution increases. Which of the following equations represents the reaction that occurs as the titration proceeds?
| A.
|
|
| B.
|
|
| C.
|
|
| D.
|
Explanation
During a titration, a titrant (ie, a reactant solution with a known concentration) is slowly added to a solution containing an unknown concentration of an analyte (ie, a species to be detected or measured). A reaction that occurs between the titrant and the analyte is used to detect and calculate the amount of the analyte that is present.
In a redox titration, the reaction that occurs between the titrant and the analyte is an oxidation-reduction (redox) reaction. The equivalence point occurs when all the analyte in the solution has reacted. This can be detected either by using an electrode to monitor the solution potential (voltage) or by an indicator that changes color when the reaction is completed.
During the redox reaction described in this question, the K2Cr2O7(aq) in the titrant solution oxidizes the C2H5OH(l), causing it to decompose into CO2(g) and H2O(l). Because the titration is performed in an acidic solution (ie, with an excess of H+ ions) and the pH is observed to increase, some of the H+(aq) ions must be consumed as reactants during the reaction. Among the four equation choices, only Choice B shows H+(aq) being consumed.
(Choice A) This reaction incorrectly shows H+(aq) being produced from the redox titration. Production of H+(aq) would cause the pH to decrease.
(Choice C) Although this reaction would lead to an increase in pH because OH−(aq) is being produced, this equation is for a redox titration in a basic, rather than an acidic, solution.
(Choice D) This reaction incorrectly has OH−(aq) being consumed and H2O(l) being produced. This would cause a decrease in pH, not an increase.
Things to remember:
In a redox titration, an oxidation-reduction (redox) reaction occurs between the titrant and the analyte. Redox titrations can be done in acidic, basic, or neutral solutions and may cause pH changes.
Question
NH3(aq) + HCl(aq) → NH4Cl(aq)
Two aqueous solutions containing 3.0 g of ammonia (NH3) and 6.4 g of hydrochloric acid (HCl), respectively, are mixed in a beaker, and the reaction shown above goes to completion. The water is evaporated, leaving crystals of solid ammonium chloride (NH4Cl). The crystals are placed on weigh paper and weighed, and a mass of 9.7 g is recorded. If each of the following errors occurred during the experiment, which would not help account for the excess mass?
| A. Some of the water was not evaporated and remained in the sample. | |
| B. The mass of the weigh paper was not determined prior to weighing the sample. | |
| C. The molar mass of ammonium chloride was not calculated correctly. | |
| D. The beaker was not cleaned properly prior to use in the experiment. |
Explanation
The law of conservation of mass states that atoms in a chemical reaction cannot be created or destroyed, only rearranged. Because each atom has a fixed mass, the mass of the reactants must be equal to the mass of the products.
In the experiment, 9.4 g of reactants (3.0 g of ammonia (NH3) and 6.4 g of hydrochloric acid (HCl)) were mixed. Because the reaction went to completion, the mass of the resulting ammonium chloride (NH4Cl) product should be 9.4 g. However, the measured mass of the crystals was 9.7 g, so 0.3 g must have been contributed by one or more sources other than the NH4Cl produced by the reaction. These sources may have been introduced by the following errors:
- Incomplete evaporation of the water, which causes a small amount of water to remain in the crystals and contribute additional mass (Choice A).
- Failure to account for the mass of the weigh paper, causing the mass of the paper itself to be included in the final measurement (Choice B).
- Failure to properly clean the beaker prior to the experiment, which causes any contaminants in the beaker to contribute additional mass to the products (Choice D).
The question asks which of the errors would not contribute to the excess mass. An incorrect calculation of the molar mass of NH4Cl could contribute to an incorrect calculation of the number of moles of NH4Cl obtained, but measured quantities such as the mass of the crystals are independent of calculated quantities such as the molar mass of NH4Cl.
Things to remember:
Atoms in a chemical reaction are neither created nor destroyed. Therefore, the mass of the reactants in a reaction equals the mass of the products.

Study On The Go
Tackle AP Chemistry Unit 4 practice test questions on the ride home from a game, review a quiz on chemical reactions between classes, or dive into your study guide while waiting for friends at the coffee shop. Keep everything you need to succeed on your AP Chem test right at your fingertips with the app. Download now!
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chemistry Unit 4 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 4: Chemical Reactions?
AP Chemistry Unit 4 focuses on understanding and mastering chemical reactions. Key topics include:
- Introduction to reactions: The basics of how and why chemical reactions occur.
- Net ionic equations: How to simplify reactions to show only the species that change.
- Representations of reactions: Using chemical equations, symbols, and diagrams to convey reactions.
- Physical and chemical changes: Differentiating between changes in matter and changes in composition.
- Stoichiometry: Calculations involving reactants and products, including limiting reactants and percent yield.
- Types of chemical reactions: Synthesis, decomposition, single and double replacement, and combustion reactions.
Covering each topic ensures you are prepared for AP Chemistry Unit 4 MCQs and FRQs. For structured review, UWorld’s AP Chemistry Unit 4 study guide, practice tests, and progress check FRQs provide detailed solutions and step-by-step explanations. You can try a free trial to explore videos, quizzes, and practice problems before committing, giving you a complete and exam-ready study plan.
How should I prepare for an AP Chemistry Unit 4 exam?
Effective preparation requires a balance of concept review and practice tests. Begin with your AP Chemistry Unit 4 study guide to understand the concepts behind chemical reactions. Then, reinforce learning with multiple-choice questions and quizzes to strengthen your understanding. Don’t forget FRQs—practicing unit 4 AP Chemistry FRQs helps improve analytical and problem-solving skills.
- Alternate between multiple-choice questions and FRQs for full coverage.
- Use unit 4 AP Chemistry practice tests to simulate real exam conditions.
- Track your answers to identify weak areas and focus your review efficiently.
UWorld provides a complete AP Chemistry Unit 4 prep platform with video lessons, practice tests, and quizzes, allowing you to study anytime and review step-by-step solutions. Start with a free trial to see how structured practice boosts your confidence and performance on the test.
Are any free resources available for AP Chemistry Unit 4?
Yes! There are several free resources to support your AP Chemistry Unit 4 review. Khan Academy offers lessons on chemical reactions, stoichiometry, and solution chemistry, while YouTube channels like Tyler DeWitt and Bozeman Science provide clear, topic-specific videos. The College Board’s AP Classroom also has sample questions to help you gauge your understanding.
While these free tools are helpful for supplemental learning, pairing them with UWorld ensures a more structured and efficient approach. Their platform offers interactive video lessons, practice questions, and FRQs designed specifically for Unit 4, helping you identify weak areas and reinforce key concepts. Start with a free trial to experience how targeted practice and detailed explanations can make your study sessions more effective and boost your confidence for the exam.
What types of questions are on the AP Chemistry Unit 4 test?
For AP Chemistry Unit 4, the exam evaluates your knowledge and application of chemical reactions through both multiple-choice and free-response questions.
MCQs (Unit 4): Test understanding of chemical reactions, stoichiometry, types of reactions, and interpreting data from experiments or diagrams.
FRQs (Unit 4): Assess application of reaction concepts, designing and analyzing experiments, calculating yields, representing reactions with equations or graphs, and supporting claims with evidence.
These questions are designed to evaluate both conceptual knowledge and problem-solving skills for Unit 4. Practicing with UWorld’s AP Chemistry Unit 4 MCQs, quizzes on chemical reactions, and FRQs provides step-by-step explanations and helps build confidence for exam day. Start with a free trial to explore targeted practice and interactive lessons.
What is the weight of AP Chemistry Unit 4 on the exam?
AP Chemistry Unit 4 accounts for an important portion of the exam, focusing on chemical reactions, stoichiometry, and reaction types. Questions from this unit appear in both the multiple-choice and FRQ sections, and together they typically make up about 7%–9% of the total exam score.
Understanding the weight of Unit 4 can help you prioritize study time and focus on the concepts most likely to appear. Multiple-choice questions test your ability to analyze chemical reactions, interpret data from experiments or diagrams, and solve stoichiometry problems. Free-response questions evaluate your skills in designing and analyzing experiments, calculating yields, representing reactions with equations or graphs, and supporting scientific claims with evidence.
To prepare effectively, practice using UWorld’s Unit 4 practice tests and quizzes on chemical reactions. Their platform provides step-by-step explanations for every question, helping you identify weak areas and reinforcing your understanding of high-priority topics. Start with a free trial to explore interactive lessons, targeted practice, and FRQs that will boost your confidence and performance on the exam.
What are common mistakes students make in AP Chem Unit 4?
Students often make several common mistakes in AP Chemistry Unit 4, which can significantly impact their performance. One frequent error is misidentifying types of chemical reactions, such as confusing single replacement with double replacement or failing to recognize a combustion reaction. Mistakes in stoichiometry calculations and determining limiting reactants are also widespread, often resulting in incorrect product amounts or percent yield calculations.
Other pitfalls include overlooking the difference between physical and chemical changes, miswriting net ionic equations, or misinterpreting diagrams, tables, and experimental data in progress check MCQs, chemical reactions quizzes, and FRQs. Students sometimes fail to organize their work in FRQs, skip showing calculations, or neglect to include supporting evidence when justifying claims, which can lead to lost points even if their conceptual understanding is correct.
The best way to avoid these mistakes is consistent, targeted practice. UWorld’s Unit 4 practice tests, quizzes on chemical reactions, and FRQs provide step-by-step solutions and explanations, helping students identify and correct errors. Starting with a free trial allows you to explore interactive lessons, build accuracy, and gain confidence, ensuring you are well-prepared for all aspects of the AP Chemistry Unit 4 exam.
How do I self-study for AP Chemistry Unit 4 effectively?
Self-studying for AP Chemistry Unit 4 can be highly effective if you follow a structured approach. Begin by reviewing your Unit 4 study guide and class notes to reinforce your understanding of chemical reactions, stoichiometry, types of reactions, net ionic equations, and physical versus chemical changes. Make sure to read through examples and worked problems to see how concepts are applied in practice.
Next, incorporate practice questions and tests into your study routine. Work through unit 4 AP Chemistry practice tests, quizzes on chemical reactions, and FRQs to simulate exam conditions and identify areas where you need improvement. Alternate between multiple-choice questions and free-response questions to ensure you are confident with both formats.
Additionally, use interactive resources like videos and animations to visualize reaction mechanisms and concepts, which can help make abstract ideas more concrete. Track your answers carefully and review explanations for every mistake, as this is key to improving accuracy and understanding.
UWorld offers a comprehensive platform for self-study, providing practice tests, FRQs, and quizzes with detailed step-by-step explanations. Starting with a free trial allows you to explore targeted Unit 4 content and build confidence, making your independent study more effective and exam-ready.
How can I improve my score on the Free-Response Questions (FRQs) for Unit 4?
Improving your score on AP Chemistry Unit 4 FRQs requires both understanding the concepts and practicing the exam format. Start by thoroughly reviewing key topics like chemical reactions, stoichiometry, reaction types, net ionic equations, and physical versus chemical changes. Make sure you understand how to apply these concepts to experimental scenarios and data analysis.
Practice is critical. Work through Unit 4 AP Chemistry FRQs, progress check FRQs, and chemical reactions quizzes under timed conditions to simulate the exam. Focus on organizing your answers clearly, showing all calculations, labeling diagrams correctly, and justifying every claim with evidence. Reviewing step-by-step solutions helps you understand common pitfalls and strengthens your problem-solving approach.
Additionally, refine your skills in representing data through graphs, tables, and chemical equations, as this is frequently tested on FRQs. Practicing both long and short response questions ensures you are confident across all FRQ formats.
UWorld offers targeted Unit 4 FRQs, quizzes on chemical reactions, and detailed explanations that guide you through each step of solving a problem. Starting with a free trial allows you to practice efficiently, identify weaknesses, and develop strategies to boost your FRQ score for the exam.
Where can I find a good study guide for AP Chemistry Unit 4?
A highly recommended study guide for AP Chemistry Unit 4 focuses on chemical reactions, stoichiometry, net ionic equations, and types of chemical reactions. The best guides include clear explanations, practice questions, and review exercises that reinforce key concepts.
Printable or digital guides often provide learning objectives, example problems, and review questions to help you track your understanding of the material. Interactive guides with practice problems and quizzes allow you to apply concepts in real exam-style scenarios.
For structured self-study, using UWorld’s AP Chemistry Unit 4 study guide is particularly effective. It combines practice tests, FRQs, quizzes on chemical reactions, and video lessons to provide step-by-step explanations. Starting with a free trial lets you explore the platform and ensures you are fully prepared for Unit 4 content on the exam.
Can I find practice tests specifically for AP Chem Unit 4?
You can find practice tests specifically for AP Chemistry Unit 4 through several resources, but for the most effective preparation, UWorld is a top choice. UWorld offers a comprehensive suite of Unit 4 practice tests, quizzes on chemical reactions, and FRQs, all designed to mirror the actual AP exam in format and difficulty. Each question comes with step-by-step explanations, allowing you to understand why each answer is correct or incorrect and helping you identify and correct common mistakes. This makes it easier to build a strong conceptual foundation and improve problem-solving skills.
In addition to UWorld, Khan Academy provides lessons and exercises on chemical reactions, stoichiometry, and reaction types, offering a solid supplement for reviewing concepts. The College Board also offers sample questions and unit guides that reflect the exam style, giving students a sense of what to expect on test day.
Using UWorld alongside these free resources allows for targeted, efficient practice. You can track your progress, focus on areas that need improvement, and simulate real exam conditions with timed Unit 4 practice tests. Starting with a free trial gives you access to all these interactive tools, helping you gain confidence and maximize your score on both MCQs and FRQs.
What are Unit 4 topics most frequently tested on the AP Chemistry exam?
The most frequently tested topics from AP Chemistry Unit 4 revolve around chemical reactions, stoichiometry, types of reactions, net ionic equations, and physical versus chemical changes. Questions often assess your ability to analyze reaction data, predict products, calculate yields, and balance chemical equations. Understanding these core concepts is crucial because they form the foundation for more advanced topics in later units.
Commonly tested subtopics include:
- Reaction types: synthesis, decomposition, single replacement, double replacement, and combustion reactions.
- Stoichiometry and limiting reactants: calculating amounts of reactants and products.
- Net ionic equations: identifying spectator ions and simplifying chemical equations.
- Physical vs. chemical changes: distinguishing between reaction types and properties.
- Representations of reactions: using diagrams, graphs, and symbolic equations to interpret chemical phenomena.
To master these frequently tested areas, consistent practice is essential. UWorld offers Unit 4 practice tests, quizzes on chemical reactions, and FRQs that target these high-priority topics, complete with detailed step-by-step explanations. Starting with a free trial lets you identify weak areas, reinforce critical concepts, and confidently prepare for both multiple-choice and free-response questions on the AP Chemistry exam.
What is the best way to review AP Chemistry Unit 4 before the AP exam?
The best way to review AP Chemistry Unit 4 before the AP exam is to combine concept review, practice questions, and timed assessments. Start by revisiting your Unit 4 study guide, class notes, and textbook examples to ensure you understand chemical reactions, stoichiometry, reaction types, net ionic equations, and physical versus chemical changes.
Next, reinforce your learning with practice. Work on Unit 4 MCQs, progress check MCQs, chemical reactions quizzes, and FRQs to simulate real exam conditions. Alternate between multiple-choice and free-response questions to build speed, accuracy, and confidence. Pay attention to areas where you make repeated mistakes and review explanations carefully.
For structured, interactive practice, UWorld is highly effective. Their Unit 4 practice tests, quizzes on chemical reactions, and FRQs provide detailed, step-by-step explanations and allow you to track your progress. Starting with a free trial gives you access to video lessons, practice problems, and targeted feedback, ensuring that your review is focused, efficient, and exam-ready.
Do AP Chemistry Unit 4 topics appear again in later units?
Yes, AP Chemistry Unit 4 topics often appear again in later units because chemical reactions, stoichiometry, and reaction types form the foundation for more advanced concepts. For example, understanding reaction mechanisms and limiting reactants from Unit 4 is essential when studying thermodynamics, kinetics, equilibrium, and electrochemistry in later units. Similarly, skills in writing net ionic equations and distinguishing physical versus chemical changes are used repeatedly in problem-solving and FRQs throughout the course.
To reinforce these foundational skills and ensure long-term retention, consistent practice is key. UWorld provides Unit 4 practice tests, quizzes on chemical reactions, and FRQs that not only help you master this unit but also strengthen the skills you’ll apply in future units. Starting with a free trial allows you to review step-by-step explanations, track progress, and build confidence that extends beyond Unit 4.