AP® Chemistry Unit 3 Review and Practice Test
Master the fundamentals of AP® Chemistry Unit 3: Properties of Substances and Mixtures with our complete review. This unit explores how the structure and intermolecular forces of solids, liquids, and gases determine their properties and behavior. You’ll study kinetic molecular theory, solutions and mixtures, and gain insight into spectroscopy and photon interactions used to analyze substances. Use our AP Chem Unit 3 review to connect theory with application through detailed lessons, study notes, and practice tests that mirror the AP exam.
Boost Your Confidence and Score High with Our AP Chemistry Unit 3 Review
Explore AP Chemistry Unit 3: Properties of Substances and Mixtures with everything you need to master the topic. This review breaks down complex ideas like intermolecular forces, solids, liquids, and gases, and kinetic molecular theory into simple, visual explanations, all part of our comprehensive AP Chem Unit 3 review. Learn how solutions and mixtures behave under different conditions and connect these concepts to real-world applications through spectroscopy and photon-based analysis. Practice with AP Chemistry Unit 3 multiple choice questions (MCQs) and free response questions (FRQs) that mirror the real exam, and reinforce your understanding with engaging study materials and step-by-step lessons.
Engaging Video Lessons
Challenging concepts like intermolecular forces, kinetic molecular theory, and phase changes are simplified through clear, visual lessons that make learning intuitive. Our expert-led videos explain how solids, liquids, and gases behave based on molecular interactions and how spectroscopy reveals the structure of substances. Learn faster with concise, high-yield videos tailored for AP Chemistry Unit 3 students studying the Properties of Substances and Mixtures.
Interactive Study Guides
Our AP Chemistry Unit 3 study guide covers every concept outlined in the College Board’s framework for Properties of Substances and Mixtures. Explore how intermolecular forces (IMFs) influence the behavior of solids, liquids, and gases, and how kinetic molecular theory explains temperature and motion at the particle level. Review solutions and mixtures, study phase changes, and understand how spectroscopy and photon interactions help identify substances. Built-in knowledge checks and visuals reinforce learning, making this guide your go-to tool for AP Chem Unit 3 progress check MCQs and exam preparation.
Try These AP Chemistry Unit 3 Practice Test Questions
Question
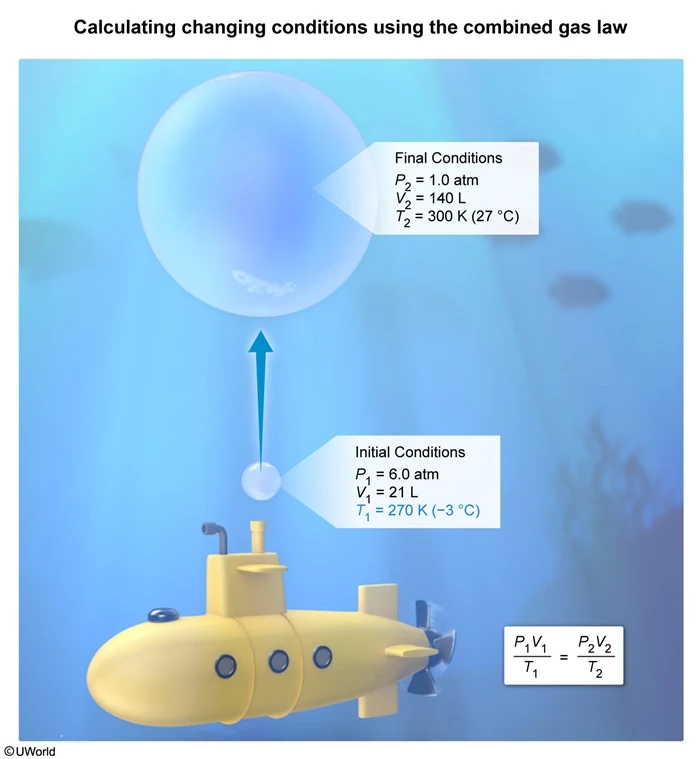
A bubble of air with a volume of 21 L is released from a submarine at an ocean depth where the pressure is 6.0 atm. If the bubble expands to a volume of 140 L as it rises near the ocean surface where the temperature is 27 °C and the pressure is 1.0 atm, what is the temperature of the saltwater at the ocean depth where the bubble is released?
| A. −3 °C | |
| B. 7.5 °C | |
| C. 24 °C | |
| D. 30 °C |
Explanation
An ideal gas is a hypothetical gas with well-behaved (idealized) characteristics used to make a simplified model of the behavior of real gases. Following this model, the behavior of a gas can be described by the ideal gas law:
where P is the pressure, V is the volume occupied, n is the number of moles of gas, T is the absolute temperature (Kelvin), and R is a constant. This equation can be rearranged as
For processes in which P, V, and T change but n does not, the initial conditions (indicated by the subscript 1) can be related to the final conditions (indicated by the subscript 2) by the combined gas law:
or simply
In this question, the initial conditions are P1 = 6.0 atm, V1 = 21 L, and T1 = ?, and the final conditions are P2 = 1.0 atm, V2 = 140 L, and T2 = 27 °C (300 K). Solving the combined gas law for T1 and substituting the condition values into the equation yields:
The temperature in Celsius must be converted to Kelvin by adding 273 to give
Without a calculator, this can be solved by factoring and canceling terms
Converting this temperature to Celsius by subtracting 273 yields an initial temperature of −3° C.
(Choice B) A temperature of 7.5 °C results from the P1/P2 ratio being inverted as P2/P1 during the calculation.
(Choice C) An initial temperature of 24 °C results if T2 (27 °C) is not converted to Kelvin prior to the calculation.
(Choice D) A temperature of 30 °C results from both the P1/P2 and V1/V2 ratios being inverted and from T2 not being converted to Kelvin prior to the calculation.
Things to remember:
When the moles of gas in a system remain constant, the ideal gas law can be expressed as the combined gas law to evaluate gas behavior under changing conditions of pressure, volume, and absolute temperature.
Question
2 NI3(s) → N2(g) + 3 I2(g)
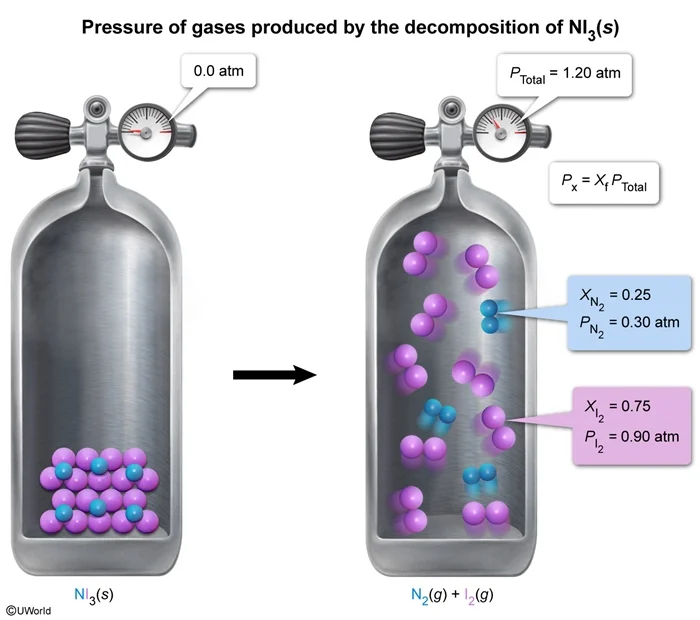
A sample of NI3(s) is placed within a rigid, previously evacuated reaction vessel and fully decomposed at a temperature of 20 °C, according to the equation above. After the reaction, the pressure inside the vessel is 1.20 atm. What is the pressure exerted by the I2(g) in the vessel?
| A. 0.30 atm | |
| B. 0.60 atm | |
| C. 0.90 atm | |
| D. 1.20 atm |
Explanation
Dalton's law of partial pressures states that the total pressure PTotal in a vessel containing a mixture of gases is equal to the sum of the partial pressure Px of each component gas within the vessel.
The partial pressure of a gas can be determined using its mole fraction Xf (ie, the moles of component gas nx divided by the total number of moles nTotal).
In the decomposition of NI3(s) described in the question, the sample is placed within a rigid, previously evacuated reaction vessel (ie, no air in the vessel). As a result, the only gases in the vessel at the end of the reaction are the products N2(g) and I2(g). The reaction equation shows that each reaction cycle produces 4 moles of gas. This means that the final product mixture is 25% N2(g) and 75% I2(g), as indicated by the mole fraction of each gas.
Multiplying the total pressure by the mole fraction of I2(g) gives the partial pressure of I2(g):
(Choice A) A pressure of 0.30 atm is exerted by the N2(g).
(Choice B) The difference in the pressures exerted by the N2(g) and I2(g) is 0.60 atm. Based on the mole ratio of the reaction, the pressure exerted by the I2(g) must be 3 times greater than the pressure exerted by the N2(g).
(Choice D) The total pressure in the vessel is 1.20 atm, which is the sum of the partial pressures exerted by the N2(g) and I2(g), as demonstrated by:
Things to remember:
The pressure of an ideal gas does not depend on the identity of the gas. At a given temperature and volume, the total pressure of a gas mixture is equal to the sum of the partial pressure exerted by each component gas, which is proportional to its mole fraction.
Question
Which of the following techniques would allow for separation of a mixture of two liquids based on intermolecular interactions with mobile and stationary phases?
| A. Distillation | |
| B. Column chromatography | |
| C. Filtration | |
| D. Titration |
Explanation
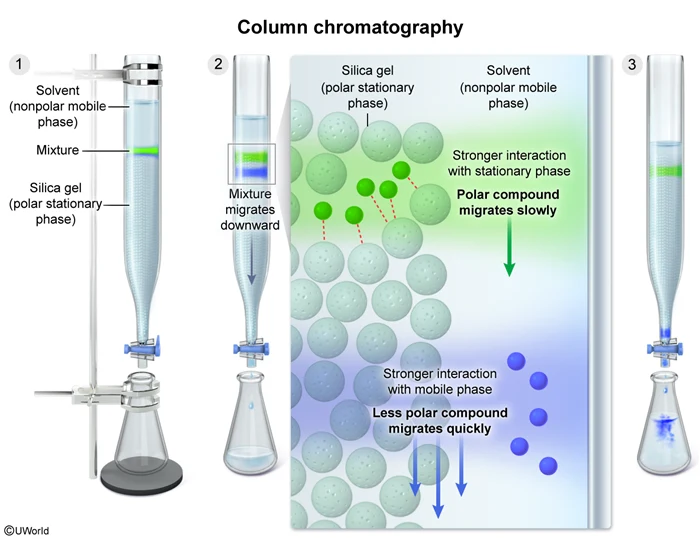
Chromatography (eg, column chromatography, thin-layer chromatography, or paper chromatography) is a technique that uses a stationary phase and a solvent (a mobile phase) to separate the components of a mixture according to their polarity. Separation occurs because the mixture components travel at different rates, depending on the strength of the competing intermolecular interactions between the mixture component and the stationary and mobile phases.
For example, when a mixture is separated by column chromatography using a polar stationary phase and a nonpolar mobile phase, a more polar compound will travel slowly down the column because it has stronger intermolecular interactions with the stationary phase than with the mobile phase. Conversely, a less polar compound will travel quickly down the column because it has stronger intermolecular interactions with the mobile phase than with the stationary phase.
Therefore, column chromatography can be used to separate a mixture of two liquids based on intermolecular interactions with a stationary and mobile phase.
(Choice A) Although distillation can be used to separate a mixture of two liquids, distillation does not have a mobile and a stationary phase. Instead, it is based on boiling points and intermolecular forces between the molecules of the mixture.
(Choice C) Filtration is a physical process used to separate a solid from a liquid, and cannot be used to separate a mixture of liquids.
(Choice D) Titration is a technique used for acid-base neutralization.
Things to remember:
Separation of a mixture using chromatography occurs because the mixture components travel through the column at different rates, depending on the strengths of the competing intermolecular interactions between each component and the stationary phase and mobile phase.

Study Anywhere, Anytime
Practice AP Chemistry Unit 3 questions wherever you are; on your commute, between classes, or at your favorite café. Watch quick concept videos on intermolecular forces or phase changes, or review notes on kinetic molecular theory in minutes. With the UWorld app, your AP Chemistry review of Properties of Substances and Mixtures fits right in your pocket; study smarter, faster, and on your schedule.
Stand Out
with a Top Score in AP Chemistry
Finish your Unit 3 AP Chemistry review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What is AP Chemistry Unit 3 about?
AP Chemistry Unit 3: Properties of Substances and Mixtures explores how the structure and forces between particles determine the physical and chemical behavior of matter. This unit builds your understanding of intermolecular forces, phase changes, and solution behavior, linking microscopic interactions to observable properties. You’ll study how molecules attract, repel, and organize themselves in solids, liquids, and gases, and how these patterns explain melting points, solubility, and vapor pressure. In Unit 3 AP Chem, students explore how structure, bonding, and energy relationships shape measurable chemical properties and behavior.
A strong grasp of these principles is essential for success in later topics like thermodynamics, equilibrium, and kinetics, where particle motion and energy relationships are key.
UWorld’s AP Chemistry Unit 3 study guide provides detailed explanations, colorful visuals, and worked examples that make abstract concepts tangible. Combined with exam-style practice questions and video lessons, it helps you connect theory to application and confidently approach unit 3 Chem questions on the AP exam.
Which topics are covered in AP Chemistry Unit 3?
AP Chemistry Unit 3 covers the foundational concepts that explain how different substances interact and transition between phases. The major topics include:
- Intermolecular Forces: London dispersion, dipole–dipole, hydrogen bonding, and ion-dipole interactions that influence boiling point, viscosity, and solubility.
- Solids, Liquids, and Gases: Structure, compressibility, and how molecular arrangement affects phase changes and density.
- Kinetic Molecular Theory: Relationship between particle motion, pressure, and temperature in gases.
- Solutions and Mixtures: Concentration, solubility, and the effects of IMFs on miscibility.
- Spectroscopy: How energy absorption and emission reveal molecular identity and structure.
Together, these topics build your ability to connect microscopic particle behavior with macroscopic chemical observations.
UWorld’s AP Chemistry QBank, aligned with College Board’s CED, includes targeted Unit 3 practice questions and custom quizzes for each subtopic, ensuring you can apply theory through data-driven, exam-style problems.
Where can I find the best AP Chemistry Unit 3 study guide and notes?
The best AP Chemistry Unit 3 study guide combines conceptual clarity with application practice. UWorld’s study guide for properties of substances and mixtures, offers complete coverage of every topic, from intermolecular forces and phase changes to solutions and spectroscopy. Each section includes step-by-step explanations, clear visuals, and real-world examples that simplify complex particle interactions. You’ll also find knowledge checks that ensure you understand how IMFs, molecular structure, and phase behavior connect to physical properties.
Unlike traditional study materials, UWorld’s emphasizes active learning. You’ll encounter knowledge checks after each section, ensuring you fully understand how IMFs, molecular structure, and phase behavior connect to physical properties. The digital version of the guide is also available in the UWorld app, allowing you to access flashcards, progress tracking, and concise AP Chemistry Unit 3 notes anytime. You can also access the AP Chemistry Unit 3 review PDF for quick print reference.
For students looking to supplement classroom materials or the College Board’s CED, UWorld provides the most reliable, exam-aligned Unit 3 AP Chemistry resource.
Where can I find AP Chemistry Unit 3 practice tests with detailed explanations?
The College Board offers free-response questions for AP Chemistry, but multiple-choice explanations are not provided; this is where UWorld fills the gap. UWorld’s AP Chemistry Unit 3 practice tests feature both MCQs and FRQs designed to mirror the real exam’s format and difficulty. Each question in UWorld’s QBank is designed with the same analytical rigor you’ll find in official progress checks, making it the ideal resource for students revisiting the Unit 3 progress check MCQ AP Chemistry answers to clarify concepts and strengthen reasoning.
You can also create custom quizzes focused on specific Unit 3 topics like IMFs, kinetic molecular theory, or spectroscopy. After each session, detailed performance analytics help you identify weak areas and refine your strategy. By practicing consistently in UWorld’s platform, you’ll gain deeper insight into molecular interactions, master exam timing, and confidently apply these AP Chemistry principles on test day.
What types of questions are usually featured on the AP Chemistry Unit 3 test?
The AP Chemistry unit 3 test includes a mix of multiple-choice and free-response questions that test both conceptual and quantitative understanding. You’ll encounter MCQs that ask you to compare intermolecular forces, predict phase changes, or identify properties of solids, liquids, and gases based on particle diagrams. Other questions test your grasp of kinetic molecular theory, such as calculating pressure or interpreting graphs showing molecular motion.
Free-response questions often involve analyzing real data, like interpreting spectroscopy results, explaining energy changes during phase transitions, or connecting IMFs to boiling point and solubility trends. Expect to justify reasoning with equations, diagrams, and scientific vocabulary. Practicing these question types using UWorld’s AP Chemistry Unit 3 MCQs and FRQs builds both speed and accuracy, helping you connect microscopic particle behavior with macroscopic observations.
What AP Chemistry Unit 3 concepts should students master before the test?
Before your AP Chemistry Unit 3 test, make sure you thoroughly understand the core principles that define how substances interact and transition between phases. Focus on these high-priority areas:
- Intermolecular Forces: Identify and compare London dispersion, dipole–dipole, hydrogen bonding, and ion–dipole interactions.
- Kinetic Molecular Theory: Relate temperature, pressure, and particle speed to gas behavior.
- Phase Changes: Analyze heating curves, vapor pressure, and energy changes during melting, freezing, and boiling.
- Solutions and Mixtures: Calculate concentration, solubility, and effects of temperature on dissolving.
- Spectroscopy: Interpret absorption and emission spectra to determine molecular properties.
A strong grasp of these AP Chemistry Unit 3 concepts connects molecular structure to macroscopic behavior and forms the foundation for later topics like thermodynamics and equilibrium. Solidifying these skills ensures you’re ready for any AP Chem unit 3 test or FRQ challenge.
UWorld’s visual explanations and problem sets help reinforce each topic through step-by-step reasoning and immediate feedback.
Are any free resources available for AP Chemistry Unit 3?
Yes! UWorld offers a free trial that gives you access to AP Chemistry Unit 3: Properties of Substances and Mixtures practice questions, detailed explanations, and visual learning tools. The trial lets you explore topics like intermolecular forces, phase changes, and solutions through realistic, exam-style questions. Each item includes a step-by-step rationale, making even complex ideas like kinetic molecular theory easier to grasp.
The free trial is designed to give students a hands-on preview of UWorld’s full learning experience; combining visuals, equations, and conceptual reasoning in one platform. It’s a great way to strengthen understanding and experience high-quality AP Chemistry Unit 3 practice materials before subscribing.
How can I find an AP Chemistry Unit 3 cheat sheet or quick reference guide?
While a complete review is always ideal, UWorld’s concise summaries and quick reference sheets work perfectly as an AP Chemistry unit 3 cheat sheet for last-minute study. Our materials highlight the most important concepts and formulas so you can review quickly before a test or quiz. You’ll find:
- Key explanations of intermolecular forces, their relative strengths, and how they affect melting points, boiling points, and solubility.
- A summary of phase changes, including how energy is absorbed or released during melting, vaporization, condensation, and sublimation.
- Simplified notes on solids, liquids, and gases, with emphasis on structure, motion, and particle energy.
- Essential details on solutions and mixtures, covering solubility rules, polarity effects, and concentration formulas.
- A quick overview of kinetic molecular theory and how it explains gas pressure, temperature, and motion.
- Brief points on spectroscopy, showing how absorption and emission data reveal molecular identity and energy changes.
However, remember that an AP Chemistry unit 3 cheat sheet should supplement, not replace your main study routine. Use these tools for rapid reviews between study sessions, and rely on UWorld’s detailed practice questions and explanations for deeper understanding of properties of substances and mixtures.
What are the best flashcards for AP Chemistry Unit 3 review?
The most effective AP Chemistry Unit 3 flashcards reinforce essential vocabulary, key formulas, and conceptual relationships central to mastering the Properties of Substances and Mixtures. UWorld’s flashcards are built for active recall and spaced repetition, ensuring you retain complex chemistry concepts long-term while improving application skills. Each deck includes:
- Core vocabulary terms such as intermolecular forces, dipole–dipole interaction, hydrogen bonding, vapor pressure, and molarity, helping you build precise scientific language.
- Explanations of intermolecular forces (IMFs); their relative strengths, polarity, and influence on boiling points, solubility, and surface tension.
- Key ideas from kinetic molecular theory, showing how molecular motion determines energy, pressure, and phase behavior.
- Details on phase changes, describing how energy is absorbed or released during melting, vaporization, condensation, and sublimation.
- Summaries of solutions and mixtures, including solubility principles, polarity interactions, and concentration formulas.
- Brief notes on spectroscopy, highlighting how light absorption and emission data reveal molecular identity and energy levels.
UWorld’s digital flashcard system uses adaptive spaced repetition to strengthen recall exactly when you need it. You can tag difficult terms, monitor progress, and link cards directly to AP Chemistry unit 3 practice questions, creating a seamless, efficient study experience.
How can I maximize my AP Chemistry Unit 3 review?
A strong AP Chemistry unit 3 review blends concept learning, problem-solving, and visual reinforcement. Start by revisiting key theories like intermolecular forces, kinetic molecular theory, and the relationships among solids, liquids, and gases. Review how molecular structure impacts observable properties such as boiling point, solubility, and vapor pressure.
Once you’ve reviewed, focus on application; solve practice problems, interpret spectroscopy data, and analyze phase change diagrams. UWorld’s integrated platform helps you read, watch, and practice together through concise study guides, clear video explanations, and exam-style AP Chemistry unit 3 MCQs and FRQs. This structured approach ties microscopic reasoning to macroscopic outcomes efficiently. A complete AP Chem unit 3 review that blends reading, watching, and practice enhances both understanding and test-day performance.