AP® Chemistry Unit 1 Review and Practice Test
From electrons to energy levels, the AP® Chemistry Unit 1 covers the foundation for everything that follows. Whether you’re just starting your AP Chem Unit 1 review or reviewing the structure of atoms and electron configuration, UWorld gives you everything you need - videos, guides, and practice questions that mirror the exam to help you score a 5.
Build Exam Endurance by Mastering the Basics of Atomic Structure
The journey to scoring a 5 in AP Chem starts with Unit 1. Here’s where you learn how protons, neutrons, and electrons define matter properties and interact with each other. The first step to mastering the unit is to use resources that help you simplify even the most complex topics with visuals, examples, and in-depth explanations that actually make sense. With UWorld’s AP Chemistry Unit 1 review, you not only master the concepts but also build exam readiness skills and lay a solid foundation.
Bringing Atomic Theory to Life
Our short and focused videos bring you an up-close understanding of electronics, orbitals, and energy levels. Experts lead each AP Chemistry Unit 1 video lesson with clear visuals and real explanations, so you understand not just what, but “why” behind each concept. Watching these lessons isn’t just review; it’s mastering the language of chemistry.
Simplifying Atomic Structure with Interactive Study Guides
Forget those dreaded long textbooks, as UWorld’s AP Chem Unit 1 study guide complements the video series to break down everything in easy, digestible lessons. From trends, equations, and atomic models in color-coded diagrams, the guides help you retain more in less time, so you review faster, understand deeper, and remember longer.
Test Your Chemistry With Real AP-Style Questions
Question
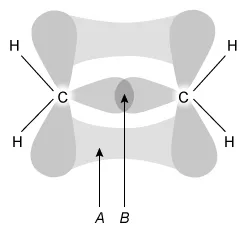
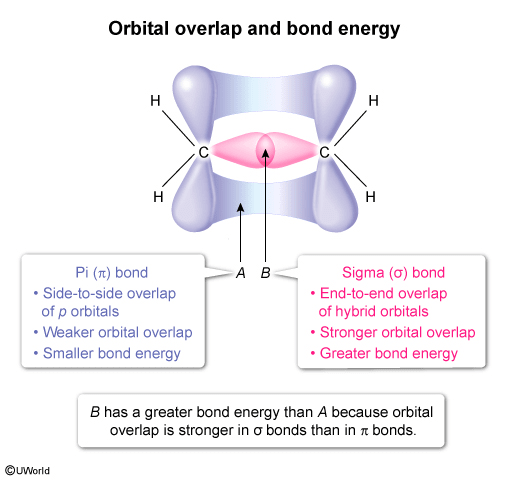
The orbital overlap forming the sigma and pi bonds that make up the C=C bond in C2H4 is shown above. Compared to the bond energy of A, the bond energy of B is
| A. the same, because the orbital overlap is the same strength | |
| B. greater, because the orbital overlap is weaker in sigma bonds than in pi bonds | |
| C. greater, because the orbital overlap is stronger in sigma bonds than in pi bonds | |
| D. smaller, because the orbital overlap is stronger in sigma bonds than in pi bonds |
Explanation
Sigma (σ) bonds are covalent bonds that result from end-to-end overlap of hybrid orbitals, which are formed from a combination of s and p atomic orbitals. Pi (π) bonds result from the side-to-side overlap of p orbitals. Orbital overlap σ bonds stronger than in π bonds, resulting in a stronger bond and greater bond energy (ie, more energy is required to break a σ bond than a π bond).
In this question, the depiction of C2H4 shows the orbital overlaps that form the σ and π bonds in the C=C double bond. The bond labeled A depicts side-to-side overlap of p orbitals and is therefore a π bond. The bond labeled B depicts end-to-end overlap of sp2 hybrid orbitals and is therefore a σ bond. As a result, the bond energy of B is greater than that of A because the orbital overlap is stronger in σ bonds than π bonds.
(Choice A) The bond energy of σ and π bonds cannot be the same because their orbital overlaps are not the same strength.
(Choices B) The bond energy of B is greater than A because orbital overlap is stronger in σ bonds than in π bonds.
(Choices D) Stronger orbital overlap results in greater bond energy rather than smaller bond energy.
Things to remember:
Sigma (σ) bonds result from end-to-end overlap of hybrid orbitals (combination of s and p atomic orbitals), whereas pi (π) bonds result from side-to-side overlap of p orbitals. σ bonds have stronger orbital overlap than π bonds, which results in σ bonds having greater bond energy than π bonds.
Passage:
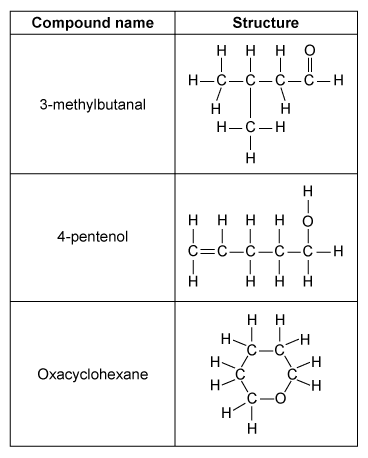
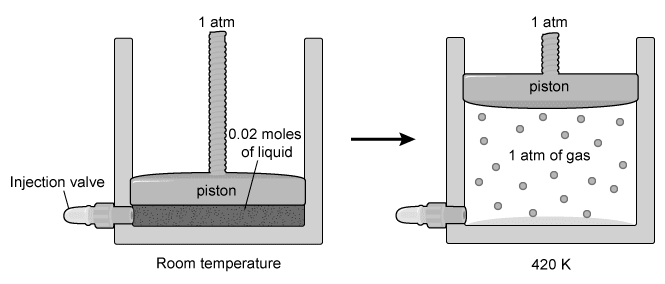
The three molecules shown in the table above are liquids at room temperature, and each have the molecular formula C5H10O. A 0.02 mole sample of each compound is separately injected into three identical containers, each with a movable piston. The compounds are vaporized by heating to 420 K, causing each piston to rise to keep the internal pressure equal to atmospheric pressure (1 atm), as illustrated below.
Question
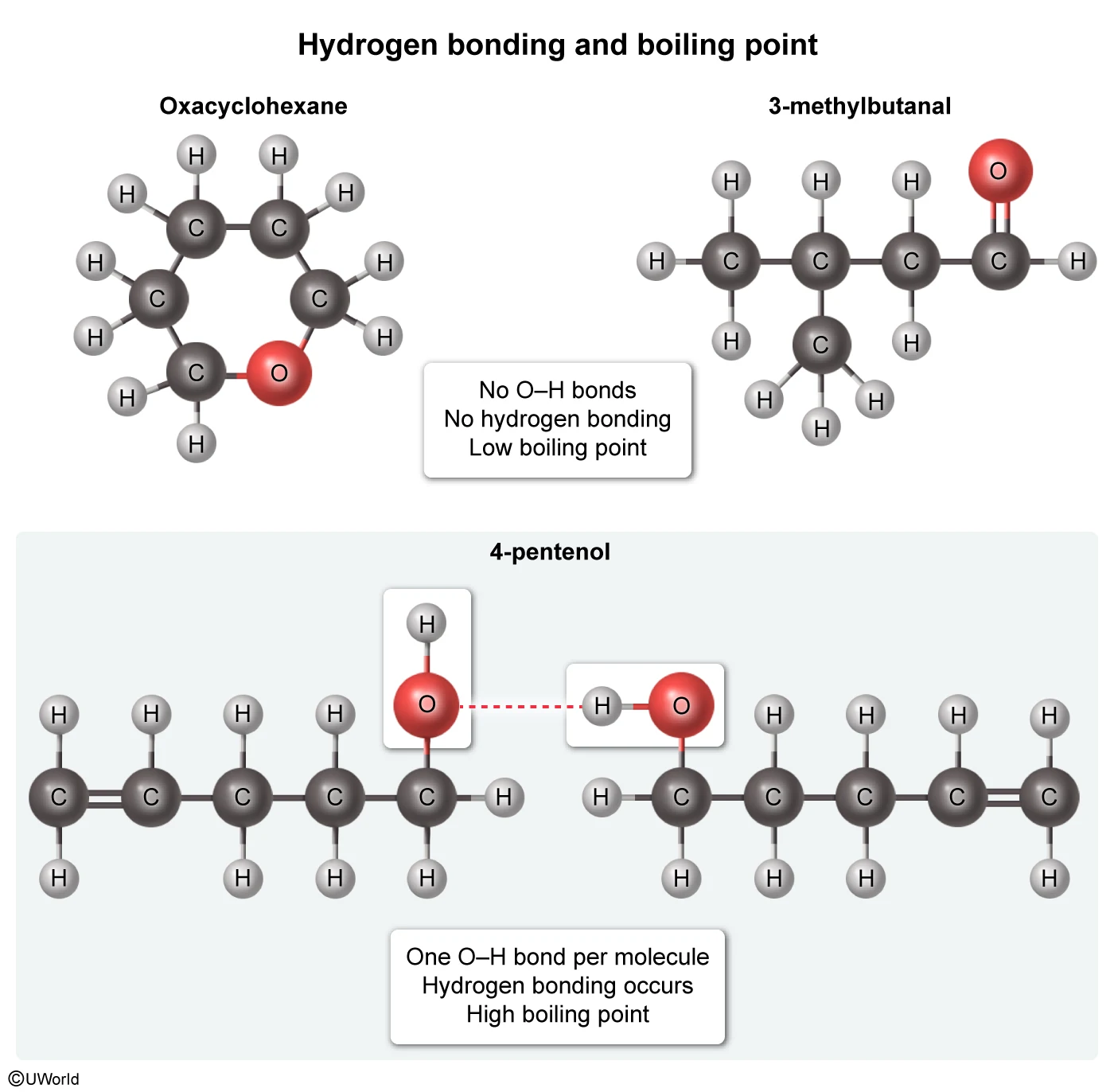
Oxacyclohexane and 3-methylbutanal each boil near 90 °C, but 4-pentenol boils at 141 °C. Which of the following interactions best explains why the boiling point of 4-pentenol is so much higher than the boiling points of the other molecules?
A. 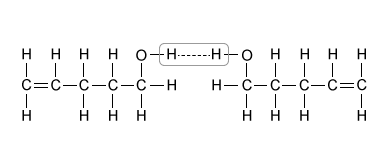 |
|
B. 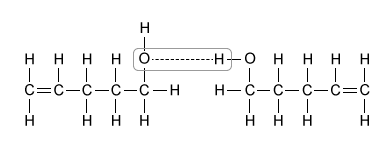 |
|
C. 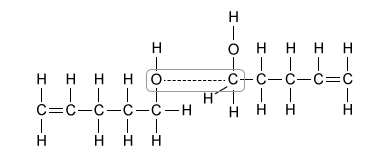 |
|
D. 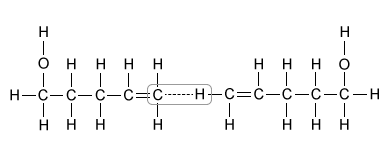 |
Explanation
The boiling point of a compound is determined by the strength of the intermolecular forces holding the molecules together. A compound boils when its thermal energy is sufficient to break these interactions, so the stronger the forces, the higher the boiling temperature.
All three molecules in the table have the same molecular formula (C5H10O), but the arrangement of the atoms differs in each, resulting in different intermolecular interactions. Hydrogen bonds are among the strongest intermolecular forces, and are capable of forming between an N, O, or F atom with a lone electron pair and a hydrogen atom that is covalently bound to a different N, O, or F atom.
Of the molecules in the table, only 4-pentenol can hydrogen bond with itself. This hydrogen bonding, depicted in Choice B, significantly increases the boiling point of 4-pentenol relative to the other compounds, and it accounts for the large difference in boiling points.
(Choice A) Hydrogen bonds occur between an H atom and an N, O, or F atom. They do not occur between two H atoms, as depicted in this option.
(Choice C) Interactions between carbon and oxygen can occur in all three molecules shown in the table, so they are unlikely to account for the difference in boiling point.
(Choice D) Interactions between carbon and hydrogen are examples of London dispersion forces, not hydrogen bonds. London dispersion forces are the weakest intermolecular forces and are present in all three molecules in the table to similar extents, so they most likely do not account for the boiling point difference.
Things to remember:
The boiling point of a compound depends on the strength of the intermolecular forces holding the molecules together. Strong forces such as hydrogen bonds yield higher boiling points than weaker forces such as London dispersion forces.
Passage:
An average sample of a pure, unidentified element is analyzed by mass spectrometry and five isotopes are detected, as listed in the table below.
| Isotope | Mass (amu) | Relative Abundance (mass percent) |
|---|---|---|
| 1 | 90 | 51.34% |
| 2 | 91 | 11.22% |
| 3 | 92 | 17.15% |
| 4 | 94 | 17.38% |
| 5 | 96 | 2.80% |
Question
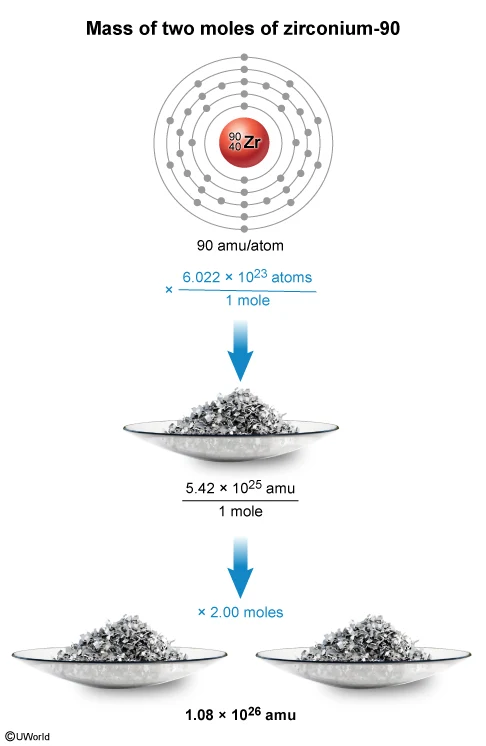
The mass of 2.00 moles of isotope 1 is approximately equal to
| A. 2.99 × 10−22 amu | |
| B. 1.80 × 102 amu | |
| C. 1.20 × 1024 amu | |
| D. 1.08 × 1026 amu |
Explanation
In a sample of a substance, individual particles of matter (ie, atoms, ions, molecules) are too small to be seen and counted separately. Instead, the relationship between the number of particles and their mass must be used. This relationship is provided by Avogadro's number (6.022 × 1023), which is the number of atomic mass units (amu) equal to a mass of exactly 1 gram.
6.022 × 1023 amu = 1.000 gram
Based on this relationship, a counting unit called the mole is defined as:
1 mole = 6.022 × 1023 particles
Accordingly, the number of atoms in 2.00 moles of isotope 1 is calculated as:
The table states that each atom of isotope 1 has a mass of 90 amu. Therefore, the mass (in amu) of 1.20 × 1024 atoms (2.00 moles) of isotope 1 is approximately equal to:
(Choice A) 2.99 × 10−22 amu is obtained if 2.00 moles is divided by Avogadro's number instead of multiplying during the first step of the calculation.
(Choice B) 1.80 × 102 amu is the mass of only 2 atoms of isotope 1. This value results from multiplying the isotope mass by 2 instead of multiplying by Avogadro's number.
(Choice C) 1.20 × 1024 amu is calculated if the number of atoms of isotope 1 is not multiplied by 90 amu (ie, effectively assumes that the atoms have a mass of 1 amu).
Things to remember:
The number of particles in a sample is related to the sample mass through Avogadro's number (6.022 × 1023), which is defined as 1 mole.

Study On Your Schedule
Whether you’re commuting or waiting at the lab bench doesn’t matter. You can review orbital diagrams, answer periodic trend questions, or watch a quick video on AP Chem Unit 1 with one click. Every lesson and question is perfectly synced across devices with UWorld’s mobile app, so you never lose your progress.
Stand Out
with a Top Score in AP Chemistry
Finish your AP Chemistry Unit 1 review and continue mastering all units with UWorld. Boost your performance and make yourself a standout candidate for competitive colleges, majors, and scholarships by earning a top score.
Get our all-in-one course today!
- Focused AP Chem Videos
- Print & Digital Study Guide
- 400+ Exam-style Practice Questions
- Customizable Quiz Generator
- Adjustable Study Planner
- Realistic Timed Test Simulation
- Colorful Visual Explanations
- Progress Dashboard
- Smart Flashcards
- Digital Notebook
Hear From Our AP Students

UWorld’s service is pretty good and helps provide a lot of explanations on subjects I haven’t been confident on before.

The questions here are the most realistic to the AP tests I've seen so far! I appreciate the ability to customize tests as well.

The best part is that all options are well-explained, telling clearly why they are not the right option.
Frequently Asked Questions (FAQs)
What topics are included in AP Chemistry Unit 1: Atomic Structure and Properties?
The AP Chem Unit 1 introduces fundamental chemistry concepts, such as the structure of atoms, the interaction of subatomic particles, and the periodic trends that drive chemical properties. It’s the basis of what you’ll learn in the following table, as you look at how electrons occupy orbitals and atomic radii change across the table. Here are the core topics:
- Moles and molar mass
- Elemental composition of pure substances
- Atomic structure and electron configuration
- Mass spectra of elements
- Composition of mixtures
- Periodic trends
- Valence electrons and ionic compounds
How should I prepare for an AP Chemistry Unit 1 exam?
Preparing for the AP Chemistry Unit 1 ideally will help you master other units as well. Start by having conceptual clarity, not through rote memorization, but through visualizing and practicing them. Watch short videos explaining each trend or equation, then test your understanding with targeted practice. Here’s what the best approach looks like:
- Review video lessons to grasp complex concepts
- Summarize formulas and periodic patterns using flashcards
- Take timed practice tests for UWorld’s AP Chem Unit 1 to simulate exam pacing
With UWorld’s AP Chem Unit 1 review, you don’t have to memorize concepts; you’ll learn to apply and master them for the exam day.
Are any free resources available for AP Chem Unit 1?
Yes. UWorld offers a 7-day free trial that gives you access to the video lessons, interactive study guides, and in-depth explanations that power your understanding and practice on the integrated platform. You may find free resources elsewhere covering the “what.” However, UWorld goes beyond that and teaches you the “why” behind each concept so you can apply them meaningfully.
What types of questions are on the AP Chemistry Unit 1 test?
Each conceptual and quantitative question on the AP Chem Unit 1 tests multiple skill levels and your ability to connect atomic structure to observed chemical behaviour. You’ll see questions requiring you to identify trends, justify reasoning, or interpret data from graphs and tables. Here’s the typical question format:
- Multiple-choice questions
- Free-response questions
- Data-based reasoning tasks
Having robust and targeted practice across these formats helps you build exam readiness and the reasoning skills needed to move beyond memorization and earn a 5.
How can I improve my score on the Free-Response Questions (FRQs) for Unit 1?
The key to a strong performance in FRQs is exactly what UWorld specifically trains you with: its in-depth explanations. These questions require you to focus on not just what, but more importantly, the why, by focusing on reasoning with evidence and structure. Here are some tips for maximizing your FRQ score:
- Ensure you use the established conventions and chemical terms instead of vague language.
- Support every conclusion with data trends and atomic principles, while also reasoning out other factors.
- Practice writing concise and direct explanations.
UWorld’s AP Chem Unit 1 FRQ examples show you exactly how to think like an AP grader and make it easier to score points.
What is the "Atomic Structure and Properties" unit's weight on the AP Chemistry exam?
Unit 1 accounts for 7-9% of the exam score in AP Chem. While it’s an early unit, its importance extends throughout the course, with topics such as periodic trends, valence electrons, and ionic compounds. A strong foundation in this unit gives you an edge across multiple units and boosts your overall AP Chem score.
What is the best way to review AP Chemistry Unit 1 before the AP exam?
You can review the AP Chem Unit 1 with short videos for a visual refresher by focusing on the concepts and how they connect. Then, use the study guide to solidify your understanding, followed by practice questions that reinforce the learning and help you strengthen your weak areas. The strategy is reflected in the UWorld’s review, which includes the watch, read, and practice philosophy for scoring a 5 on the AP Exam.
Here’s a quick pre-exam checklist:
- Review periodic trends and electron configurations
- Revisit difficult FRQs and note recurring patterns
- Use study guides to enforce key formulas and concepts
- Use timed quizzes to work on speed and accuracy under pressure
Do AP Chemistry Unit 1 topics appear again in later units?
Absolutely. The AP Chem Unit 1 covers fundamental concepts that act as the backbone of chemistry. Each concept, like ionization energy, atomic size, and mixture composition, appears in different units. Understanding these relationships early makes complex topics far easier to grasp later. Every minute spent mastering Unit 1 saves hours of review later.