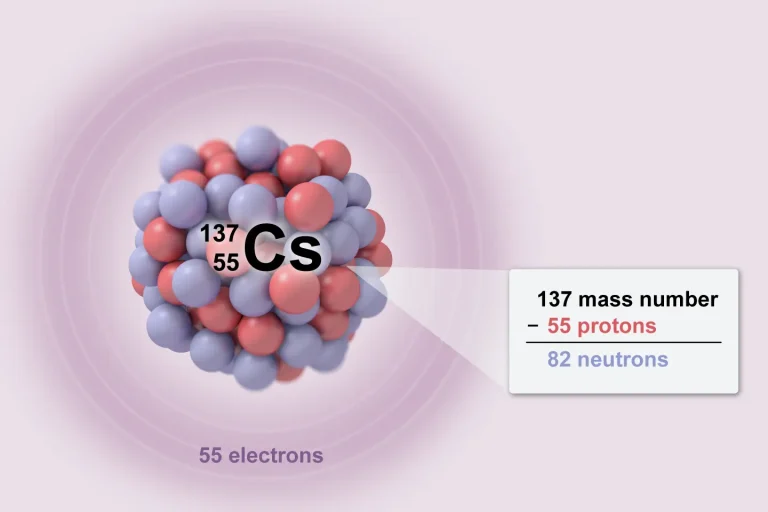
AP Chemistry provides a solid foundation in college-level chemistry. In 2025, 168,833 students took the exam. Here’s an overview of the AP Chemistry units and topics to help you prepare for the AP Chemistry exam.
AP Chemistry Curriculum Framework
The AP Chemistry course is structured into 2 primary sections: Science Practices and Course Content. The Course Content is divided into 9 units that encompass essential AP Chemistry topics. Each unit is designed around 6 Science Practices and various AP Chemistry key concepts, which are crucial for developing a solid understanding of the subject. With exam questions closely aligned to these units and practices, a focused study plan is essential. Using an AP Chemistry online course will provide comprehensive resources to reinforce these concepts and enhance exam readiness.
AP Chemistry Science Practices
AP Chemistry includes 6 Science Practices essential for mastering the course and enhancing critical thinking and analytical abilities.
Here are the 6 Science Practices and the skills you will develop:
1. Models and Representations
- Form detailed explanations using models
- Describe properties at particulate and macroscopic levels
2. Question and Method
- Pose scientific questions and determine methods
- Develop hypotheses, identify experimental procedures, and recognize errors
3. Representing Data and Phenomena
- Create graphs, models, and diagrams
- Represent relationships across different levels and scales
4. Model Analysis
- Analyze and interpret models
- Explain connections between particulate and macroscopic properties
5. Mathematical Routines
- Solve problems using mathematical relationships
- Balance equations and use graphs for problem-solving
6. Argumentation
- Develop scientific claims and support them with data
- Use models, theories, and mathematical routines to justify claims and explain experimental results
These practices are integrated into each unit to help you learn and practice effectively.
Big Ideas of the AP Chemistry Course
There are 4 big ideas that serve as the foundation of the course, enabling students to connect concepts and develop deeper conceptual understanding.
- Scale, Proportion, and Quantity: Quantities in chemistry are expressed a both macroscopic and atomic scales, and relationships exist both within and between these 2 scales.
- Structure and Properties: Properties of substances observable at the macroscopic level emerge from the structures of atoms and molecules and the interactions between them.
- Transformations: Chemistry is about the rearrangement of matter at both macroscopic and submicroscopic scales.
- Energy: Energy plays an important role in characterising and controlling chemical systems.
AP Chemistry Units and Topics
AP Chemistry is divided into 9 units, each focusing on 1 or more of the 4 Science Practices. These units cover various AP Chemistry key concepts, topics, and science practices essential for mastering the course. For a structured review of these topics, refer to the AP Chemistry Study Guide.
Unit 1: Atomic Structure and Properties
(Exam Weight: 7%-9%)
Unit 1 covers the atomic theory of matter, explaining chemical substance properties. You'll study macroscopic systems composed of numerous atoms and molecules, learning to use moles to connect macroscopic measurements to atomic quantities. You'll also explore the periodic table's organization, atomic structure, electron configuration, and periodicity, including patterns in properties and reactivity. Access 400+ practice questions with our AP Chemistry Question Bank to improve your skills and boost your confidence.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 1.1 Moles and Molar Mass | Learn to use dimensional analysis and the mole concept to calculate the number of atoms or molecules in a given substance amount. | 5. Mathematical Routines |
| 1.2 Mass Spectra of Elements | Learn to explain quantitatively how an element’s mass spectrum relates to the masses of its isotopes. | 5. Mathematical Routines |
| 1.3 Elemental Composition of Pure Substances | Quantitatively explain how an element’s mass spectrum relates to its isotope masses. | 2. Question and Method |
| 1.4 Composition of Mixtures | Identify a sample as a pure substance or mixture based on its qualitative or quantitative composition. | 5. Mathematical Routines |
| 1.5 Atomic Structure and Electron Configuration | Use the Aufbau principle to represent the electron configurations of atoms and ions. | 1. Models and Representations |
| 1.6 Photoelectron Spectroscopy | Use the photoelectron spectrum to determine an atom or ion's electron configuration, and the energy and number of electrons in a subshell. | 4. Model Analysis |
| 1.7 Periodic Trends | Explain how the electronic structures of elements determine atomic properties and periodic trends across the periodic table. | 4. Model Analysis |
| 1.8 Valence Electrons and Ionic Compounds | Understand how periodic trends influence the reactivity and chemical properties of elements across the periodic table. | 4. Model Analysis |
Unit 2: Compound Structure and Properties
(Exam Weight: 7%-9%)
Unit 2 explores the relationship between the structure and properties of substances, including chemical bonds, electronegativity, and intermolecular forces.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 2.1 Types of Chemical Bonds | Explore how the properties of elements are influenced by the type of bond formed between them. | 6. Argumentation |
| 2.2 Intramolecular Force and Potential Energy | Learn about the factors influencing the strength of interactions between atoms and describe these interactions in terms of potential energy relative to atomic distance. | 3. Representing Data and Phenomena |
| 2.3 Structure of Ionic Solids | Learn to create or interpret a particulate model of an ionic solid based on Coulomb’s Law. | 4. Model Analysis |
| 2.4 Structure of Metal and Alloys | Learn to create or interpret models of metallic solids and alloys, highlighting key structural features and interactions. | 4. Model Analysis |
| 2.5 Lewis Diagrams | Learn to make or interpret Lewis diagrams that represent molecules. | 3. Representing Data and Phenomena |
| 2.6 Resonance and Formal Charge | Learn to create or interpret Lewis diagrams illustrating resonance between equivalent structures or facilitating the use of formal charge to determine the most stable configuration among nonequivalent structures. | 6. Argumentation |
| 2.7 VSEPR and Bond Hybridization | Explain a molecule’s structural and electron properties using VSEPR theory and Lewis diagrams. | 6. Argumentation |
Unit 3: Properties of Substances and Mixtures
(Exam Weight: 18%-22%)
Unit 3 explores how the properties of solids, liquids, and gases are determined by particle arrangement, mobility, and interactions.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 3.1 Intermolecular and Interparticle Forces | Describe how well a model or representation explains the link between properties at the particle level and observable, large-scale properties. | 4. Model Analysis |
| 3.2 Properties of Solids | Explain how a substance’s macroscopic properties depend on its particle-level structure and interactions. | 4. Model Analysis |
| 3.3 Solids, Liquids, and Gases | Use particle diagrams and models to explain the differences between solid, liquid, and gaseous states of matter. | 3. Representing Data and Phenomena |
| 3.4 Ideal Gas Law | Use the ideal gas law to determine the properties of a gas or gas mixture at the macroscopic level. | 5. Mathematical Routines |
| 3.5 Kinetic Molecular Theory | Use kinetic molecular theory, particulate models, and graphs to explain how particle movement affects a gas's macroscopic properties. | 4. Model Analysis |
| 3.6 Deviation from Ideal Gas Law | Explain how interactions and particle volumes cause non-ideal gas behavior and identify conditions that promote it. | 6. Argumentation |
| 3.7 Solutions and Mixtures | Learn the properties of a solution and use molarity to relate the moles of solute to the volume of the solution. | 5. Mathematical Routines |
| 3.8 Representations of Solutions | Learn to use particle diagrams to represent interactions and concentrations of chemical species in solution mixtures. | 3. Representing Data and Phenomena |
| 3.9 Separation of Solutions and Mixtures | Learn how chromatography, distillation, and other separation methods exploit differences in the strength of interactions between chemical species to separate components of a solution. | 2. Question and Method |
| 3.10 Solubility | Explain chemical compound solubility in aqueous and non-aqueous solvents based on interactions between the compound and solvent particles. | 4. Model Analysis |
| 3.11 Spectroscopy and the Electromagnetic Spectrum | Learn the types of molecular and electronic transitions caused by each region of the electromagnetic spectrum. | 4. Model Analysis |
| 3.12 Properties of Photons | Explain how the energy of a photon relates to the electronic transition that occurs in an atom or molecule. | 5. Mathematical Routines |
| 3.13 Beer-Lambert Law | Use the Beer-Lambert Law to explain the relationship between light absorption in a solution and concentration, path length, and molar absorptivity. | 2. Question and Method |
Unit 4: Chemical Reactions
(Exam Weight: 7%-9%)
Unit 4 involves the making and breaking of bonds, transforming reactants into products, and altering their properties.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 4.1 Introduction for Reactions | Learn to identify the difference between physical and chemical changes in matter. | 2. Questions and Method |
| 4.2 Net Ionic Equations | Learn to write and balance full chemical equations and net ionic equations to describe changes in matter. | 5. Mathematical Routines |
| 4.3 Representations of Reactions | Learn to use particulate models to represent and describe chemical reactions or physical reactions at the molecular and atomic level. | 3. Representing Data and Phenomena |
| 4.4 Physical and Chemical Changes | Learn how changes in bond interactions affect the macroscopic properties of a substance. | 6. Argumentation |
| 4.5 Stoichiometry | Learn to use balanced chemical equations to calculate how changes in reactant quantities affect product quantities in a chemical reaction. | 5. Mathematical Routines |
| 4.6 Introduction to Titration | Learn to use titrations to find a solution’s equivalence point, indicating complete reactant consumption, and to calculate the initial amount of reactant. | 3. Representing Data and Phenomena |
| 4.7 Types of Chemical Reactions | Learn to identify chemical reactions as:
|
1. Models and Representations |
| 4.8 Introduction to Acid-Base Reactions | Learn to identify chemical species as:
|
1. Models and Representations |
| 4.9 Oxidation-Reduction (Redox) Reactions | Learn to use half-reactions to balance redox reactions, which involve the transfer of electrons. | 5. Mathematical Routines |
Unit 5: Kinetics
(Exam Weight: 7%-9%)
Unit 5 examines reaction rates based on molecular collisions, influenced by environment, temperature, reactant amounts, and catalysts.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 5.1 Reaction Rates | Learn how experimental parameters, such as temperature and reactant concentration, affect the rate of a chemical reaction. | 6. Argumentation |
| 5.2 Introduction to Rate Law | Shorten this content. But make sure to not miss out on the important points. | 5. Mathematical Routines |
| 5.3 Concentration Changes Over Time | Learn to interpret graphs of chemical concentrations vs. time to determine rate orders and formulate the rate law equation for a given reaction. | 5. Mathematical Routines |
| 5.4 Elementary Reactions | Learn to apply stoichiometry to establish the rate law expression for elementary reactions. | 5. Mathematical Routines |
| 5.5 Collision Model | Learn how the frequency, orientation, and energy of molecular collisions influence the rate of a reaction. | 6. Argumentation |
| 5.6 Reaction Energy Profile | Learn to use reaction energy profiles to illustrate a reaction's activation energy and the net change in energy from reactants to products. | 3. Representing Data and Phenomena |
| 5.7 Introduction to Reaction Mechanics | Learn how elementary reactions combine to form the reaction mechanism of a more complex, multistep reaction. | 1. Models and Representations |
| 5.8 Reaction Mechanism and Rate Law | Learn to determine the rate law for multistep reactions, especially when the rate-limiting elementary reaction is not the first step. | 5. Mathematical Routines |
| 5.9 Pre-Equilibrium Approximation |
Learn to determine the rate law for more multistep reactions whose rate-limiting elementary reaction is not the first step. | 5. Mathematical Routines |
| 5.10 Multistep Reaction Energy Profile | Learn to draw reaction energy profiles for multistep reactions, displaying the activation energy and the total energy change of the reaction. | 3. Representing Data and Phenomena |
| 5.11 Catalysis | Learn how catalysts affect reactions by altering activation energy and/or reaction mechanisms. | 6. Argumentation |
Unit 6: Thermochemistry
(Exam Weight: 7%-9%)
Unit 6 explains the role of energy in chemical reactions, predicting their direction and energy transfer.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 6.1 Endothermic and Exothermic Processes | Learn how energy changes during chemical and physical processes correlate with macroscopic observations, like temperature variations. | 6. Argumentation |
| 6.2 Energy Diagrams | Learn to utilize energy diagrams to illustrate physical or chemical alterations and the acquisition or dissipation of energy. | 3. Representing Data and Phenomena |
| 6.3 Heat Transfer and Thermal Equilibrium | Discover how molecular collisions are impacted by thermal energy availability. | 6. Argumentation |
| 6.4 Heat Capacity and Calorimetry | Learn to utilize calorimetry experiments to determine the heat absorbed or released by a chemical system. | 2. Question and Method |
| 6.5 Energy of Phase Changes | Learn about molar enthalpy during phase transitions and how heat is absorbed or released when substances transition between different states of matter. | 1. Models and Representations |
| 6.6 Introduction to Enthalpy of Reaction | Learn to calculate the molar enthalpy of a reaction based on the heat released or absorbed for a given amount of reacting substance. | 4. Model Analysis |
| 6.7 Bond Enthalpies | Learn to calculate the overall enthalpy change of a reaction based on the bond energies of the individual bonds broken or formed during the reaction. | 5. Mathematical Routines |
| 6.8 Enthalpy of Formation | Learn to use standard enthalpies of formation for products and reactants to calculate the overall enthalpy change of a reaction. | 5. Mathematical Routines |
| 6.9 Hess’s Law | Learn to represent a process as multiple steps and explain how the sum of enthalpies from each step relates to the overall enthalpy of the process. | 5. Mathematical Routines |
Unit 7: Equilibrium
(Exam Weight: 7%-9%)
Unit 7 covers reversible reactions where molecules form or break bonds, reaching equilibrium when forward and reverse reactions occur at the same rate.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 7.1 Introduction to Equilibrium | Observable processes are reversible at equilibrium. Forward and reverse reactions balance out, resulting in no net changes in the system. | 6. Argumentation |
| 7.2 Direction of Reversible Reactions | The rates of forward and reverse reactions determine the net direction of a reversible reaction. | 4. Model Analysis |
| 7.3 Reaction Quotient and Equilibrium Constant | Learn to represent Qc or Qp of a reversible reaction and how they relate to the equilibrium constant, Kc or Kp. | 3. Representing Data and Phenomena |
| 7.4 Calculating the Equilibrium Constant | Learn to calculate Kc or Kp from concentrations or pressures at equilibrium. | 5. Mathematical Routines |
| 7.5 Magnitude of the Equilibrium Constant | Learn how K value relates to the ratio of products to reactants at equilibrium. | 6. Argumentation |
| 7.6 Properties of the Equilibrium Constant | Learn to represent a multistep process with an equilibrium expression and combine individual equilibrium constants to find the overall equilibrium constant. | 5. Mathematical Routines |
| 7.7 Calculating Equilibrium Concentrations | Learn to use initial conditions, a balanced equation, and the appropriate K to identify the concentration or partial pressure of a species at equilibrium. | 3. Represent Data and Phenomena |
| 7.8 Representations of Equilibrium | Learn how to use particle diagrams to represent reversible reactions. | 3. Representing Data and Phenomena |
| 7.9 Introduction to Le Chȃtelier’s Principle | Learn how to use Le Chȃtelier’s principle to predict how a system will change in response to changes in external conditions. | 6. Argumentation |
| 7.10 Reaction Quotient and Le Chȃtelier’s Principle | Learn how to predict the direction of a reversible reaction by comparing Q and K. | 5. Mathematical Routines |
| 7.11 Introduction to Solubility Equilibria | Learn how to use a salt’s Ksp value to calculate its solubility. | 5. Mathematical Routines |
| 7.12 Common-Ion Effect | Learn how the solubility of a salt is affected by the presence of one of the ions in that salt, known as a common ion. | 2. Question and Method |
Unit 8: Acids and Bases
(Exam Weight: 11%-15%)
Unit 8 focuses on the role of water as an acid and a base in chemical equilibrium.
| Topic | Objective | Suggested Science Practice |
|---|---|---|
| 8.1 Introduction to Acids and Bases | Learn to use Kw and species concentrations in an aqueous solution to calculate pH and pOH values. | 5. Mathematical Routines |
| 8.2 pH and pOH of Strong Acids and Bases | Learn to use species concentrations in a strong acid-base solution to calculate pH and pOH. | 5. Mathematical Routines |
| 8.3 Weak Acid and Base Equilibria | Learn to explain how concentrations of species in a weak acid or base solution relate to pH and pOH. | 5. Mathematical Routines |
| 8.4 Acid-Base Reactions and Buffers | Learn to explain how concentrations of major species are related in a mixture of weak and strong acids and bases. | 5. Mathematical Routines |
| 8.5 Acid-Base Titrations | Learn to explain how titration results of acid or base solutions relate to their properties. | 5. Mathematical Routines |
| 8.6 Molecular Structure of Acids and Bases | Learn to explain how the structure of a molecule or ion dictates the strength of an acid or base. | 6. Argumentation |
| 8.7 pH and pKa | Learn how the pKa of a weak acid relates to the pKb of its conjugate base, impacting the predominant form of the acid/base at a given pH. | 2. Question and Method |
| 8.8 Properties of Buffers | Learn to calculate buffer solution pH using the acid's pKa and the concentration ratio of the acid-base pair. | 6. Argumentation |
| 8.9 Henderson-Hasselbalch Equation | Learn how to calculate the pH of a buffer solution using the pKa of the acid and the concentration ratio of the conjugate acid-base pair. | 5. Mathematical Routines |
| 8.10 Buffer Capacity | Learn to explain how buffer capacity depends on the concentrations of the conjugate acid and base in the solution. | 6. Argumentation |
| 8.11 pH and Solubility | Learn to explain how the solubility of a salt is affected by changes in pH. | 2. Question and Method |
Unit 9: Thermodynamics and Electrochemistry
(Exam Weight: 7%-9%)
Unit 9 applies thermodynamics principles to determine if a reaction occurs, its direction, and completion.
| Topics | Objective | Suggested Science Practice |
|---|---|---|
| 9.1 Introduction to Entropy | Learn to identify the magnitude of an entropic change during a physical or chemical process. | 6. Argumentation |
| 9.2 Absolute Entropy and Entropy Change | Learn to calculate entropy change in a process using absolute entropies of chemical species. | 5. Mathematical Routines |
| 9.3 Gibbs Free Energy and Thermodynamic Favorability | Learn to explain how the thermodynamic favorability of a process is related to ∆G°. | 6. Argumentation |
| 9.4 Thermodynamic and Kinetic Control | Learn to use kinetics to explain why a thermodynamically favorable reaction may proceed slowly. | 6. Argumentation |
| 9.5 Free Energy and Equilibrium | Learn how K, ∆G°, and T relate to the thermodynamic favorability of a reaction. | 6. Argumentation |
| 9.6 Free Energy of Dissolution |
Learn to explain how dissolution of a salt changes the enthalpy and entropy of a system. | 4. Model Analysis |
| 9.7 Coupled Reactions | Learn to explain how external energy sources or coupling with favorable reactions can drive thermodynamically unfavorable processes. | 4. Model Analysis |
| 9.8 Galvanic (Voltaic) and Electrolytic Cells | Learn to relate the operational principles of an electrochemical cell to its physical components. | 2. Question and Method |
| 9.9 Cell Potential and Free Energy | Learn to assess the thermodynamic favorability of an electrochemical reaction using the standard cell potential and the involved half-reactions. | 5. Mathematical Routines |
| 9.10 Cell Potential Under Nonstandard Conditions | Learn to explain how changes in cell conditions can cause the observed potential of an electrochemical cell to differ from the standard potential. | 6. Argumentation |
| 9.11 Electrolysis and Faraday’s Law | Learn to use Faraday’s Law to calculate electron flow rate in an electrochemical cell based on reactant consumption or product formation rates. | 5. Mathematical Routines |
AP Chemistry Labs
In AP Chemistry, labs comprise at least 25% of class time, with over 16 experiments to complete. Labs teach inquiry-based learning, preparing you for college.
Here are some of the AP Chemistry topics you will explore through the lab experiments you perform in your course:
- Redox titration
- Solubility
- Acid-base titration
- Buffers
- Stoichiometry
- Spectroscopy
To find out more about the lab experiments in the AP Chemistry curriculum, what they will help you learn, and how they play a role in your final AP Chemistry exam, check out our article on AP Chemistry Labs.
Key Concepts Students Learn Across AP Chemistry
AP Chemistry covers a wide range of topics, but many of the same core concepts reappear across different units. If the course feels dense at times, it helps to step back and focus on the bigger picture. These are the key concepts that connect everything you study in AP Chemistry.
- Structure of matter, including how atomic structure and electron behavior influence chemical properties
- Chemical bonding and intermolecular forces, which explain how substances form and interact
- Chemical reactions and stoichiometry, focusing on how matter and charge are conserved
- Energy changes in chemical processes, including thermodynamics and reaction favorability
- Reaction rates and equilibrium, which describe how and why reactions proceed or reach balance
- Acids, bases, and electrochemistry, connecting chemical behavior in solutions to real-world systems
While each unit introduces these concepts in different contexts, the goal remains the same: to help you recognize patterns, connect ideas, and apply chemistry to unfamiliar situations. Understanding how these concepts fit together makes it easier to tackle exam questions and approach new problems with confidence.

Frequently Asked Questions (FAQs)
What is the hardest unit in AP Chemistry?
Determining the toughest unit in AP Chemistry curriculum depends on individual students’ strengths and study approaches. However, based on feedback and trends from Reddit5, Units 8, 1, and 7 are often considered challenging due to their complexity and the depth of understanding required.
What are the most important topics for the AP Chemistry exam?
Based on the weighting of each unit in the exam, the most important units are:
Unit 3: Intermolecular Forces and Properties (18–22%)
Unit 8: Acids and Bases (11–15%)
Which AP Chemistry units are most heavily tested on the exam?
While all AP Chemistry units can appear on the exam, Unit 3 and Unit 8 are among the most heavily tested. These units focus on core concepts such as molecular interactions, reaction rates, and factors that affect chemical behavior, which are applied across many exam questions.
Is an equation sheet provided on the AP Chemistry exam?
Yes. The AP Chemistry exam provides a formula and constants sheet that includes commonly used equations, constants, and reference information. However, you are still expected to know when and how to apply these equations correctly, not just recognize them.
What are the AP Chemistry Science Practices?
AP Chemistry Science Practices describe the skills students are expected to demonstrate on the exam. These include analyzing data, designing and evaluating experiments, applying chemical principles, interpreting graphs and models, and explaining reasoning using scientific evidence.
Do I need to complete labs to take the AP Chemistry exam?
Completing labs is not required to sit for the AP Chemistry exam, but laboratory experience is strongly recommended. Many exam questions involve experimental design, data analysis, and interpretation of lab results, so hands-on practice helps prepare you for both multiple-choice and free-response questions.
References
AP Chemistry (About the Course). (n.d.). apstudents.collegeboard.org. Retrieved on October 25, 2024 from https://apstudents.collegeboard.org/courses/ap-chemistry
AP Chemistry (About the Exam). (n.d.). apstudents.collegeboard.org. Retrieved on October 25, 2024 from https://apstudents.collegeboard.org/courses/ap-chemistry/assessment
AP® Chemistry Course and Exam Description, Effective Fall 2024. (n.d.). apcentral.collegeboard.org. Retrieved on October 25, 2024 from https://apcentral.collegeboard.org/media/pdf/ap-chemistry-course-and-exam-description.pdf
AP Chemistry Lab Manual. (n.d.). apcentral.collegeboard.org. Retrieved on October 25, 2024 from https://apcentral.collegeboard.org/courses/ap-chemistry/classroom-resources/lab-manual
Past AP Chemistry Score Distributions. (n.d.). apstudents.collegeboard.org. Retrieved on October 25, 2024 from https://apstudents.collegeboard.org/about-ap-scores/score-distributions/ap-chemistry
AP Chemistry Updates for 2024-25. (n.d.). apcentral.collegeboard.org. Retrieved on October 25, 2024 from https://apcentral.collegeboard.org/courses/ap-chemistry/updates-2024-25
Read More About the AP Chemistry Exam
Ready to boost your chances of getting into prestigious schools? Visit our AP Chem study plan, curated by experts, and equipped with all the resources to master the exam.
AP Chemistry Exam FormatFinding it hard to grasp the AP Chemistry format? Check our article on the AP Chem structure for a clear breakdown of the structure, question types, duration, and more!
AP Chemistry Scoring GuideDiscover everything you need to know about the AP Chem scoring, including how scores are calculated and the distribution trends to help you understand your performance.
How to Self-Study for AP ChemistryAre you self-studying for AP Chemistry? Find tips and tricks on mastering concepts, tackling practice problems, and staying organized to excel on the AP Chem exam!
Best AP Chemistry Study Guide ComparisonStruggling to choose the best study guide? Compare Kaplan, Barron's, Princeton Review, and UWorld to find the perfect AP Chemistry study guide for your exam success!
Best AP Chemistry Prep Course ReviewWondering which AP Chemistry online course is the best? Check out this detailed review to find the right course for your exam prep.